文章信息
- 李冬琴,陈桂葵,郑海,黎华寿,李小兵
- LI Dong-qin, CHEN Gui-kui, ZHENG Hai, LI Hua-shou, LI Xiao-bing
- 镉对两品种玉豆生长和抗氧化酶的影响
- Effects of Cadmium on Growth and Antioxidant Enzyme Activities of Two Kidney Bean(Phaseolus vulgaris L.)Cultivars
- 农业环境科学学报, 2015, 34(2): 221-226
- Journal of Agro-Environment Science, 2015, 34(2): 221-226
- http://dx.doi.org/10.11654/jaes.2015.02.003
-
文章历史
- 收稿日期:2014-09-14
玉豆(Phaseolus vulgarisL.),又名菜豆、四季豆,系豆科菜豆属栽培种,一年生缠绕或近直立草本植物,原产美洲的墨西哥和阿根廷,我国在16世纪末开始引种栽培.玉豆营养丰富,蛋白质含量高,既是蔬菜又是粮食,还可作糕点和豆馅,是我国最重要的蔬菜种类之一,也是南菜北运最佳蔬菜之一,华南地区春秋两季均可播种.
镉(Cadmium,Cd)是生物非必需的毒性最强的重金属元素之一,具有稳定、积累和不易清除等特性,进入土壤环境中易被植物吸收,并可通过食物链进入人体,在人体内不断积累,具有潜在的致癌作用,严重威胁人体健康及生命安全[1, 2, 3].随着我国工业化的快速发展,有色金属开采、冶炼过程和使用含镉原料的工矿企业每年都有大量镉通过 “三废” 排到环境中.据研究,排到环境中的镉有82%~94%进入土壤,其中相当部分为农业土壤[4].在我国,因镉含量超标被迫弃耕的耕地约为 1.3×104hm2[5].
镉对农业环境的污染状况及其对农作物生长发育、生理特性的影响已引起人们广泛的关注[1, 6, 7, 8, 9, 10],大量研究表明,豆科植物如花生[11, 12]、大豆[13, 14]等对重金属镉有较强的耐性和富集能力,而且不同基因型(品种)间的耐性和富集能力差异明显;关于镉对玉豆的影响也有部分研究[15, 16, 17, 18],但对于镉胁迫下玉豆品种之间差异特征的研究鲜有报道.因此,本实验选用两个玉豆品种幼苗,采用水培实验,研究镉胁迫下两种玉豆生长和叶片中抗氧化酶的变化,比较它们耐镉的差异性,为探究耐镉毒害机理提供理论依据. 1 材料与方法 1.1 材料
供试材料为优选双亲16号玉豆(优选玉豆)和泽盈特选12号玉豆(特选玉豆),由广州世贸农业科技有限公司和香港泽盈农业有限公司生产. 1.2 处理方法
选择籽粒饱满的种子,用10%双氧水消毒10 min,经自来水和蒸馏水漂洗干净后,播于装有经高压灭菌的蔬菜育苗基质(由广州市园林基质厂生产)的盆钵中,待幼苗生长一周后,选择长势一致的幼苗,用海绵包茎固定幼苗,移栽到装有清水的塑料盆里(容积为1 L),加漏盖,盆外套上黑色袋子便于根系生长,每盆3株,第二天把塑料盆里的清水换成 Hoagland 全营养液,缓苗1周后,将两种玉豆分别置于镉浓度为0、2、5、8、15 mg·L-1的Hoagland营养液中培养,镉 以CdCl2·2.5H2O水溶液的形式加入营养液中.每个处理设3个重复.每隔1 d更换一次含镉营养液,1周后收获.实验于2014年3月下旬至4月中旬在广州华南农业大学一玻璃温室内自然光照条件下进行,通过空调控制其昼温为25 ℃、夜温为18 ℃. 1.3 测定方法
株高采用直接测量;植株生物量测定采用称重法,先用自来水反复冲洗干净,吸水纸吸干表面水分,称取鲜重,105 ℃下杀青30 min,于80 ℃烘干至恒重,称取干重;根形态测定使用STD-4800 根系扫描仪(Regent Instruments,Canada)进行,经WinRHIZO Reg(2009年版)根系分析软件获取根系扫描图像,并获得根系总长度、总表面积、平均直径和总体积等根构型参数.丙二醛(MDA)含量、超氧化物歧化酶(SOD)活性、过氧化物酶(POD)活性和过氧化氢酶(CAT)活性均参照邹琦[19] 的方法. 1.4 数据分析
数据处理采用Microsoft Excel 2003、SPSS 17.0统计软件,采用单因素方差分析(One-Way ANOVA)和LSD多重比较各处理间的差异显着性.作图分析采用SigmaPlot 12.0软件. 2 结果与分析 2.1 镉对两种玉豆生长特性的影响 2.1.1 玉豆株高和植株生物量
表 1显示两种玉豆的株高随着镉处理浓度的升高而显着降低.与对照相比,在2、5、8 mg·L-1和15 mg·L-1镉处理下,特选玉豆的株高分别下降了23.21%、40.05%、43.02%和56.06%,优选玉豆株高分别下降了12.32%、35.22%、37.41%和44.87%,说明相同浓度镉处理下优选玉豆的株高下降幅度小于特选玉豆.
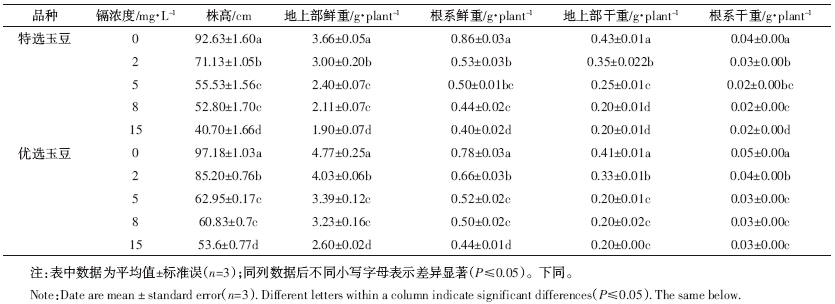 |
两种玉豆的植株生物量也随镉处理浓度的升高而明显下降,与对照相比,在2、5、8 mg·L-1和15 mg·L-1处理下,特选玉豆地上部鲜重分别下降了19.21%、34.52%、42.24%和48.87%,根系鲜重分别下降了38.3%、41.81%、48.83%和54.09%,地上部干重分别下降了18.87%、40.17%、52.78%和54.11%,根系干重下降了31.02%、35.87%、42.62%和44.17%;优选玉豆地上部鲜重分别下降了15.37%、28.96%、32.27%和45.38%,根系鲜重分别下降了15.34%、33.87%、36.74%和44.09%,地上部干重分别下降了20.26%、49.84%、51.89%和51.14%,根系干重分别下降了15.37%、35.39%、37.42%和42.67%. 2.1.2 玉豆根构型
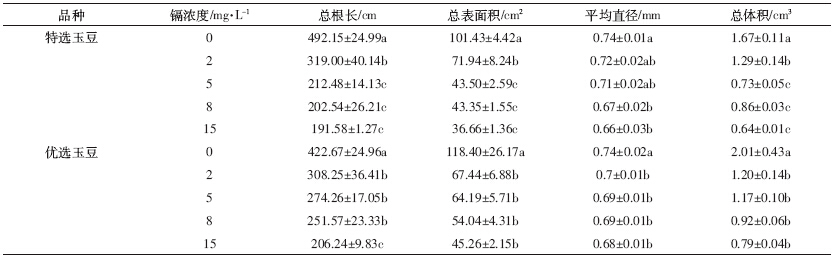
表 2显示,经不同镉浓度处理后,两种玉豆的总根长,表面积、平均直径和总体积都显着低于对照组.在2、5、8、15 mg·L-1镉处理下,特选玉豆的总根长与对照相比分别下降了35.18%、56.83%、58.85%和61.07%,优选玉豆分别下降了27.07%、35.11%、40.48%和51.21%;特选玉豆各处理组的根表面积与对照相比分别下降了29.07%、57.11%、57.26%和63.86%,优选玉豆分别下降了43.04%、45.79%、54.36%和61.77%;特选玉豆各处理组的根平均直径与对照相比分别下降了2.7%、4.05%、9.46%和10.81%,优选玉豆分别下降了5.59%、6.39%、7.39%和8.09%;特选玉豆各处理组的总根体积与对照相比分别下降了22.75%、48.5%、56.29%和61.67%,优选玉豆分别下降了40.3%、41.79%、54.23%和60.7%.其中特选玉豆的根系长度、根表面积和总体积在镉浓度为5、8、15 mg·L-1的处理组之间差异不显着;优选玉豆的根表面积、平均直径和体积在各个镉处理组之间差异不显着,其根系长度在2、5、8 mg·L-1处理组之间差异不显着.
 |
经不同浓度镉处理后,两种玉豆的MDA含量(图 1)随镉浓度的升高而显着增加,且在相同浓度下,特选玉豆体内的MDA含量明显高于优选玉豆.其中特选玉豆在镉浓度为15 mg·L-1时达到了56.62 μmol·g-1 FW,与对照相比增加了3.57倍;优选玉豆的MDA含量在15 mg·L-1时为27.72 μmol·g-1 FW,与对照相比增加了2.91倍.

|
| 图 1 镉处理对两品种玉豆MDA含量影响 Figure 1 Effect of cadmium stress on MDA contents of two kidney bean cultivars |
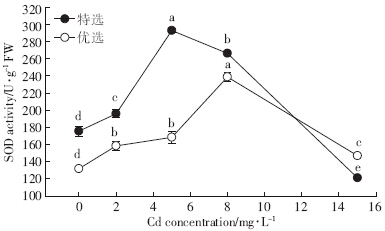
两种玉豆的SOD活性(图 2)随镉浓度的升高呈现先增加后下降的趋势.其中特选玉豆的SOD活性在各浓度间差异显着,在5 mg·L-1处理时达到最大值292.83 U·g-1 FW,与对照相比增加了66.79%,但在15 mg·L-1镉胁迫时与对照相比下降了30.95%,与5 mg·L-1处理时相比下降了58.60%;优选玉豆SOD活性在2 mg·L-1和5 mg·L-1镉处理时差异不显着,在8 mg·L-1处理时达到最大值238.78 U·g-1 FW,比对照增加了81.15%,但在15 mg·L-1镉胁迫时与对照相比增加了11.5%,与8 mg·L-1处理时相比则下降了38.48%.
|
| 图 2 镉处理对两种玉豆SOD活性影响 Figure 2 Effect of cadmium stress on SOD activity of two kidney bean cultivars |
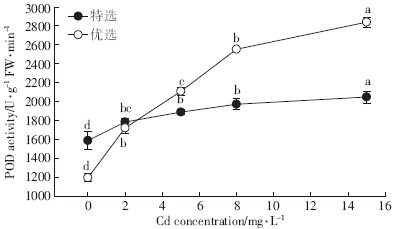
在各浓度镉处理下,两种玉豆的POD活性(图 3)都显着高于其对照组,其中特选玉豆的POD活性增加的趋势比优选玉豆缓慢且幅度小,在15 mg·L-1时达到最大值2 048.33 U·g-1 FW·min-1,比对照增加了29.1%;优选玉豆POD活性随镉浓度的升高而显着增加,在15 mg·L-1时达到最大值2840 U·g-1 FW·min-1,比对照增加了1.38倍.
|
| 图 3 镉处理对两种玉豆POD活性影响 Figure 3 Effect of cadmium stress on POD activity of two kidney bean cultivars |
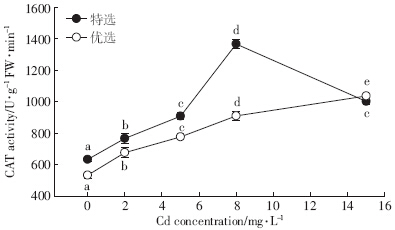
经不同镉浓度胁迫后,特选玉豆CAT活性(图 4)随胁迫浓度升高呈先增后降的趋势,且各处理与对照相比差异显着,在8 mg·L-1处理时为最高值1 366.67 U·g-1 FW·min-1,与对照相比增加了1.16倍;优选玉豆的CAT活性随胁迫浓度升高而升高,各浓度间差异显着,在15 mg·L-1处理时为最高值1 036.67 U·g-1 FW·min-1,与对照相比增加了0.94倍.
|
| 图 4 处理对两种玉豆CAT活性影响 Figure 4 Effect of cadmium stress on CAT activity of two kidney bean cultivars |
镉是一种有毒的非必需元素,易于被植物吸收、富集,过量的镉会影响植物的正常生长发育[20].有研究[21] 表明,镉胁迫下植物的株高、生物量等生长指标会受到一定的抑制作用.在本实验中,镉胁迫下两种玉豆的株高和植株质量与对照相比均明显下降,在2 mg·L-1镉胁迫时两种玉豆就开始受到明显的抑制,其抑制程度随镉胁迫浓度的升高而不断增强,在最高浓度(15 mg·L-1)时受到的抑制作用最为显着.就下降幅度而言,同样镉浓度下,特选玉豆下降的幅度大于优选玉豆,说明特选玉豆比优选玉豆更易受到重金属镉的伤害.
根系长度和表面积等指标是反应根型特征的基本参数,一般而言,植物受到外界刺激后根构型发生改变,进而影响地上部生长发育.有研究[22] 表明,钨可通过影响根系伸长区的扩张来抑制根增长.苏格兰松幼苗根系经镉处理后,根的伸长明显受到抑制[23],且镉胁迫对幼苗根尖细胞的抑制和毒害作用具有显着的积累效应[24].本研究也显现类似的结果,经镉处理后,两种玉豆的总根长、表面积、平均直径、总体积等都受到不同程度的抑制,整体而言,特选玉豆在8、15 mg·L-1处理时的降低幅度大于优选玉豆.
一般植物在重金属胁迫下体内活性氧(Reactive oxygen species,ROS)会大量增加,导致植物体内发生代谢紊乱,从而对植物的生长产生不利影响.许多研究表明镉可诱导脂质过氧化过程[25, 26],MDA是膜脂过氧化的产物,其含量可反映机体内脂质过氧化的程度,也间接反映细胞损伤的程度,是衡量膜脂过氧化损伤的指标[27].据此,通过测定植物体内MDA含量的变化,可以反映该植物抵抗不良环境能力的强弱.很多研究[28, 29] 表明,在重金属胁迫下,MDA含量上升.在本试验中,随镉胁迫浓度的增加,两种玉豆的MDA含量呈上升趋势,其中特选玉豆的上升幅度大于优选玉豆,表明特选玉豆的膜脂过氧化程度高于优选,这在一定程度上说明镉胁迫对特选玉豆的伤害程度比优选玉豆大.
为了防止脂质过氧化损伤,植物通过提高SOD、POD、CAT等各种抗氧化酶的活性来维持植物体内ROS产生和清除的动态平衡[29, 30, 31, 32].SOD酶可清除污染物诱导以及正常代谢过程产生的超氧阴离子自由基,是植物抗氧化系统发挥功能的首要酶,而CAT酶和SOD酶主要是清除植物体内的H2O2,能将H2O2分解为O2和H2O,是过氧化物酶体的主要酶类.在本实验中,两种玉豆SOD和特选玉豆CAT活性随着营养液中镉浓度的增加先上升后下降,上升的原因是植物应激产生的保护作用以降低膜脂过氧化程度,随着镉浓度的升高,镉引起的过氧化物不断积累、过氧化损伤不断增加,打破了植物体内ROS产生和清除的动态平衡,使其活性开始下降,说明两种玉豆SOD活性存在一个镉胁迫浓度的阈值.两种玉豆POD和优选玉豆CAT活性随着营养液中镉浓度的增加而持续上升,可能与两种玉豆在镉胁迫下的应激有关.从3种酶活性的变化幅度来分析,特选玉豆SOD和CAT活性的变幅大于优选玉豆,有研究表明当SOD活性降低时抗性强的品种下降幅度小[31, 32],表明优选玉豆的抗性高于特选玉豆.而优选玉豆的POD活性增强幅度远大于特选玉豆,可能是由于优选玉豆体内清除H2O2的关键酶是POD,亦或许与植物体内产生的H2O2含量有关. 4 结论
(1)随着镉处理浓度的升高,两种玉豆的株高和生物量显着降低,且特选玉豆降低的幅度大于优选玉豆.
(2)经不同浓度镉处理后,两种玉豆的总根长、表面积、平均直径和总体积都显着降低,且特选玉豆在中高浓度处理下的降低幅度大于优选玉豆.
(3)两种玉豆的MDA含量随镉浓度的升高而升高,特选玉豆MDA含量的增幅大于优选玉豆,表明其脂质过氧化程度高于优选玉豆;特选玉豆SOD活性和CAT活性变幅都大于优选玉豆,但优选玉豆POD活性变幅大于特选玉豆.
综合两种玉豆上述生长和生理指标的变化可知,优选玉豆耐重金属镉的能力高于特选玉豆.
| [1] | Sato A, Takeda H, Oyanagi W, et al. Reduction of cadmium uptake in spinach(Spinacia oleracea L.) by soil amendment with animal waste compost[J]. Journal of Hazardous Materials, 2010, 181(1-3):298-304. |
| [2] | 于方明, 刘可慧, 刘 华, 等. 镉污染对水稻不同生育期抗氧化系统的影响[J]. 生态环境学报, 2012, 21(1):88-93. YU Fang-ming, LIU Ke-hui, LIU Hua, et al. Effects of cadmium pollution on the antioxidant system of the rice in different growth period[J]. Ecology and Environmental Sciences, 2012, 21(1):88-93. |
| [3] | Stohs S J, Bagchi D, Hassoun E, et al. Oxidative mechanisms in the toxicity of chromium and cadmium ions[J]. Journal of Environmental Pathology, Toxicology and Oncology, 2001, 20(2):77-88. |
| [4] | 孟庆强, 黄国锋, 吴启堂, 等. 土壤中重金属迁移到水体的机理及预测研究[C]. 第七次""土壤与环境""学术研讨会论文摘要, 2011. MENG Qing-qiang, HUANG Guo-feng, WU Qi-tang, et al. The mechanism and prediction research of heavy metals in soil migrating to water[C]. The seventh ""Soil and environment"" academic seminar paper, 2011. |
| [5] | 李培军, 刘 宛, 孙铁珩, 等. 我国污染土壤修复研究现状与展望[J]. 生态学杂志, 2006, 25(12):1544-1548. LI Pei-jun, LIU wan, SUN Tie-heng, et al. Remedlation of contaminated soil:Its present research situation and prospect[J]. Chinese Journal of Ecology, 2006, 25(12):1544-1548. |
| [6] | Stritsis C, Steingrobe B, Claassen N. Shoot cadmium concentration of soil-grown plants as related to their root properties[J]. Journal of Plant Nutrition and Soil Science, 2012, 175(3):456-465. |
| [7] | Metwali M R, Gowayed S M H, Al-Maghrabi O A, et al. Evaluation of toxic effect of copper and cadmium on growth, physiological traits and protein profile of wheat(Triticum aestivium L. ), maize(Zea mays L. )and sorghum(Sorghum bicolor L. )[J]. World Applied Sciences Journal, 2013, 21(3):301-304. |
| [8] | Lu H P, Zhuang P, Li Z A, et al. Contrasting effects of silicates on cadmium uptake by three dicotyledonous crops grown in contaminated soil[J]. Environmental Science and Pollution Research, 2014, 21(16):9921-9930. |
| [9] | Shentu J L, He Z L, Zeng Y Y, et al. Microbial biomass and PLFA profile changes in rhizosphere of pakchoi(Brassica chinensis L.) as affected by external cadmium loading[J]. Pedosphere, 2014, 24(4):553-562. |
| [10] | Srivastava R K, Pandey P, Rajpoot R, et al. Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings[J]. Protoplasma, 2014, 251(5):1047-1065. |
| [11] | McLaughlin M J, Bell M J, Wright G C, et al. Uptake and partitioning of cadmium by cultivars of peanut(Arachis hypogaea L. )[J]. Plant and Soil, 2000, 222(1-2):51-58. |
| [12] | Shi G R, Cai Q S. Cadmium tolerance and accumulation in eight potential energy crops[J]. Biotechnology Advances, 2009, 27(5):555-561. |
| [13] | Fang X L, Zhao Y Y, Ma Q B, et al. Identification and comparative analysis of cadmium tolerance-associated miRNAs and their targets in two soybean genotypes[J]. PLoS ONE, 2013, 8(12):e81471. |
| [14] | Tang Y L, Cao Y, Qiu J B, et al. Expression of a vacuole-localized BURP-domain protein from soybean(SALI3-2) enhances tolerance to cadmium and copper stresses[J]. PLoS ONE, 2014, 9(6):e98830. |
| [15] | 吕金印, 邸丽俊, 叶庆富. 镉胁迫对菜豆幼苗基因组DNA多态性的影响[J]. 中国环境科学, 2012, 32(5):892-899. LÜ Jin-yin, DI Li-jun, YE Qing-fu. Effects of cadmium stress on DNA polymorphism of genome in Phaseolus vulgaris seedling[J]. China Environmental Science, 2012, 32(5):892-899. |
| [16] | 邸丽俊. 镉硫交互对菜豆幼苗植物络合素及DNA增色效应的影响[D]. 杨凌:西北农林科技大学, 2011. DI Li-jun. The influence of interaction between cadmium and sulfur on phytochelatin and DNA hyperchromic of phaseolus vulgaris seedling[D]. Yangling:Northwest Agriculture and Forestry University, 2011. |
| [17] | 周 青, 张 辉, 黄晓华, 等. 镧对镉胁迫下菜豆(Phaseolus vulgaris)幼苗生长的影响[J]. 环境科学, 2003, 24(4):48-53. ZHOU Qing, ZHANG Hui, HUANG Xiao-hua, et al. Effects of La on the growth of kidney bean seedling under Cd stress[J]. Environmental Science, 2003, 24(4):48-53. |
| [18] | 黄晓华, 周 青. 镧对水培菜豆和玉米幼苗镉胁迫的缓解作用[J]. 中国稀土学报, 2005, 23(2):245-249. HUANG Xiao-hua, ZHOU Qing. Lanthanum relief on hydroponic beans and corn seedling under cadmium stress[J]. Journal of the Chinese Rare Earth Society, 2005, 23(2):245-249. |
| [19] | 邹 琦. 植物生理学实验指导[M]. 北京:中国农业出版社, 2000. ZOU Qi. Plant physiology experiment guidance[M]. Beijing:China Agriculture Press, 2000. |
| [20] | 张金彪, 黄维南. 镉胁迫对草莓光合的影响[J]. 应用生态学报, 2007, 18(7):1673-1676. ZHANG Jin-biao, HUANG Wei-nan. Effects of cadmium stress on photosynthetic functions of strawberry[J]. Chinese Journal of Applied Ecology, 2007, 18(7):1673-1676. |
| [21] | Hassan M J, Shao G, Zhang G. Influence of cadmium toxicity on growth and antioxidant enzyme activity in rice cultivars with different grain cadmium accumulation[J]. Journal of Plant Nutrition, 2005, 7(28):1259-1270. |
| [22] | Adamakis I D S, Panteris E, Eleftheriou E P. Tungsten disrupts root growth in Arabidopsis thaliana by PIN targeting[J]. Journal of Plant Physiology, 2014, 171(13):1174-1187. |
| [23] | Schützendübel A, Polle A. Plant responses to abiotic stresses:Heavy metal-induced oxidative stress and protection by mycorrhization[J]. Journal of Experimental Botany, 2002, 53(372):1351-1365. |
| [24] | 李照令, 王鹤潼, 陈瑞娟, 等. 运用MSAP研究镉胁迫对拟南芥幼苗基因甲基化的影响[J]. 农业环境科学学报, 2014, 33(1):28-36. LI Zhao-ling, WANG He-tong, CHEN Rui-juan, et al. Studying genomic methylation of arabidopsis thaliana seedling under cadmium stress using MSAP[J]. Journal of Agro-Environment Science, 2014, 33(1):28-36. |
| [25] | Rahoui S, Chaoui A, El Ferjani E. Membrane damage and solute leakage from germinating pea seed under cadmium stress[J]. Journal of Hazardous Materials, 2010, 178(1-3):1128-1131. |
| [26] | Rahoui S, Ben C, Chaoui A, et al. Oxidative injury and antioxidant genes regulation in cadmium-exposed radicles of six contrasted Medicago truncatula genotypes[J]. Environmental Science and Pollution Research, 2014, 21(13):8070-8083. |
| [27] | 雷静静, 冯 佳, 谢树莲. 纳米氧化镍对3种绿藻的毒性效应[J]. 中国环境科学, 2013, 33(10):1842-1849. LEI Jing-jing, FENG Jia, XIE Shu-lian. Toxic effects of nNiO on three species of green algae[J]. China Environmental Science, 2013, 33(10):1842-1849. |
| [28] | Sfaxi-Bousbih A, Chaoui A, El Ferjani E. Unsuitable availability of nutrients in germinating bean embryos exposed to copper excess[J]. Biological Trace Element Research, 2010, 135(1-3):295-303. |
| [29] | Gusman G S, Oliveira J A, Farnese F S, et al. Mineral nutrition and enzymatic adaptation induced by arsenate and arsenite exposure in lettuce plants[J]. Plant Physiology and Biochemistry, 2013, 71:307-314. |
| [30] | Liu D L, Zhang S P, Chen Z, et al. Soil cadmium regulates antioxidases in sorghum[J]. Agricultural Sciences in China, 2010, 9(10):1475-1480. |
| [31] | 陈鸿鹏, 谭晓风. 超氧化物歧化酶(SOD)研究综述[J]. 经济林研究, 2007, 25(1):59-65. CHEN Hong-peng, TAN Xiao-feng. Literature review of researches on superoxide dismutase[J]. Nonwood Forest Research, 2007, 25(1):59-65. |
| [32] | Zhang F Q, Zhang H X, Wang G P, et al. Cadmium-induced accumulation of hydrogen peroxide in the leaf apoplast of Phaseolus aureus and Vicia sativa and the roles of different antioxidant enzymes[J]. Journal of Hazardous Materials, 2009, 168:76-84. 表1 镉对两品种玉豆株高和植株生物量的影响 |
 2015, Vol. 34
2015, Vol. 34