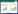文章信息
- 左海英, 张琳, 刘菲
- ZUO Hai-ying, ZHANG Lin, LIU Fei
- 大体积吹扫进样-气相色谱-同位素质谱仪测定水中苯乙烯氢同位素
- Analysis of Compound-Specific Hydrogen Isotope Compositions of Styrene in Aqueous Phases by Large Volume Injection-Gas Chromatography-Isotope Ratio Mass Spectrometry
- 农业环境科学学报, 2015, 34(5): 1017-1020
- Journal of Agro-Environment Science, 2015, 34(5): 1017-1020
- http://dx.doi.org/10.11654/jaes.2015.05.027
-
文章历史
- 收稿日期:2015-01-22
2. 中国地质科学院水文地质环境地质研究所, 河北 正定 050803
2. Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences, Zhengding 050803, China
单体同位素技术问世以来,引起了广泛关注。早在20世纪90年代,就有人利用其对沉积岩和石油中有机烃的来源进行了示踪分析[1, 2, 3]。最近的研究热点集中在监控有机污染物的迁移转化上,单体同位素分析技术不仅能够定量地表征有机污染物的转化过程,而且能够体现转化过程的机理[4, 5, 6, 7]。
某一元素的同位素分馏与其质量有关,质量小的同位素分馏大,质量大的同位素分馏小。因此,质量数为2的氢元素的同位素分馏远大于其他元素[8],比其他元素更能体现出有机物的迁移转化规律,而且氢元素与碳等元素相结合,可以更好地描绘有机物的环境行为[9, 10, 11]。国外已能进行单体氢同位素的常规分析,并在实际污染场地中应用较多[12, 13, 14, 15],但是,由于分析手段的限制,我国目前有机污染物降解过程研究中常用的是碳元素[16, 17, 18],单体氢同位素主要用于植物体或石油中的正构烷烃,以研究古环境或者揭示油田的成因等[19, 20],所以有必要开展有机污染物的单体氢同位素分析方法开发,为有机污染物的环境行为提供有效的技术支持。 1 实验部分 1.1 仪器与主要试剂
仪器:吹扫捕集仪(型号4660,美国OI公司,配置4552型自动进样器),气相色谱-燃烧-同位素质谱联用仪(气相色谱Trace GC Ultra,同位数质谱仪MAT 253,配置挥发性有机物专用色谱柱Rtx-624色谱柱,30 m×0.25 mm×1.40 μm)。
主要试剂:苯乙烯标准品(1000 mg·L-1,国家标准物质测试中心),甲醇(农残级,TEDIA公司)。 1.2 仪器条件 1.2.1 吹扫捕集仪参数
吹扫管25 mL,吹扫气氮气,吹扫时间11 min,吹扫温度40 ℃,脱附温度210 ℃,脱附时间2 min,烘焙温度220 ℃,烘焙时间5 min,传输线温度110 ℃。 1.2.2 气相色谱仪参数
色谱柱升温程序:初始温度35 ℃,保持4 min,以5 ℃·min-1升至100 ℃,保持0 min,再以20 ℃·min-1升至200 ℃,保持 2 min。
进样口温度250 ℃,载气氦气,流速1.5 mL·min-1。参考气体氢气,氢转化管的温度1450 ℃。 1.2.3 质谱仪参数
高压值9.43 mV,Trap值0.70 mA,灯电流1.5 mA。 1.3 样品处理
本实验采用标准物质配制的样品。首先用甲醇将购置的苯乙烯标准品稀释成100 mg·L-1,再配制成需要的苯乙烯水溶液,放入40 mL VOC样品瓶中,然后按照1.2所示仪器条件进行仪器设定,完成后进行样品测定。 2 结果与讨论 2.1 氢同位素分馏的影响因素
2.1.1 吹扫捕集的影响
本项目采用已经较为成熟的EPA 524方法进行吹扫捕集的条件设置,吹扫时间为11 min。由于吹扫时间足够长,目标物在捕集阱上的吸附很充分,2 min的脱附时间也能使苯乙烯从捕集阱上完全脱附出来,进入GCC-IRMS检测。因此吹扫捕集对H同位素分馏不产生影响。以1000 mg·L-1标准液直接进样和100 μg·L-1水中标准溶液吹扫捕集进行对比(两种进样方式各做3次平行),可以看出吹扫捕集不对H同位素的分馏产生影响,吹扫捕集可以用于挥发性有机物单体H同位素的前处理。具体实验结果见表 1。
常用的气相色谱仪的进行方式分为分流进样和不分流进样两种。分流进样是将液体样品注入进样口后迅速升温,使样品瞬间蒸发,在大流速载气吹扫下,样品与载气迅速混合,混合气通过分流口时,大部分混合气体被排出,只有少量的混合气体进入色谱进行分析。分流可以达到两个目的:一是减少进入毛细管色谱柱的进样量,以免使其过载;二是可以使样品出峰尖锐,有利于定量分析。
由此可知,对H这种原子量很小的元素来说,分流进样会使更多较轻的1H进入色谱柱检测,从而不可避免地产生分馏;对H进样量要求较大的同位素质谱,分流进样会大幅提高目标物的检出限。
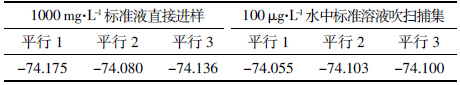
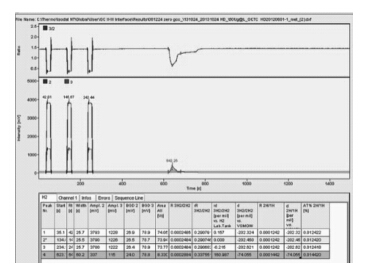
连接吹扫捕集仪的气相色谱仪推荐采用分流进样方式。由于苯乙烯中H的百分含量很低,采用最低分流比7∶1。实验结果表明:在不分流进样方式下苯乙烯中δ2H/1H值为-74.055‰,在分流进样方式下苯乙烯中δ2H/1H值为-124.404‰;气相色谱直接进样测定的标准溶液中δ2H/1H值为-74.136‰。由图 1至图 3可以看出:不分流进样时样品峰较大,但是峰型不好,前后都有延展;分流进样时样品峰明显变小,出峰尖锐。由此可知,在分流进样方式下,虽然峰型较好,但是样品峰的信号强度明显降低,尤其是H同位素发生分馏,严重影响了测定结果,因此需采用不分流进样方式。
 |
| 图 1 不分流进样色谱图 Figure 1 Chromatogram for splitless injection |
 |
| 图 2 分流进样色谱图 Figure 2 Chromatogram for split injection |
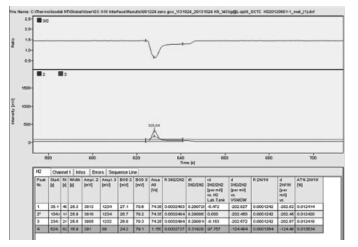 |
| 图 3 分流进样色谱峰 Figure 3 Peaks for split injection |
在40 mL样品瓶中加入目标物,使其浓度分别为10.0、30.0、50.0、60.0、80.0、100.0 μg·L-1(表 2),按照1.2进行仪器参数设置,上机测定。每个浓度考察7个平行样品,用于评价该方法对不同浓度样品测定的重复性。
根据最低检出浓度的要求(准确性和平行性),水中苯乙烯单体氢同位素的检出限定为10.0 μg·L-1,与《生活饮用水卫生标准》(GB/T 5749—2006)中苯乙烯0.50 μg·L-1的限值还有一定的距离,需要进一步降低。 2.3 实际样品测定
氢同位素不仅可以标识有机污染物的环境迁移转化过程,而且可以用于判别有机污染物的来源,即溯源。不同厂家的原料和生产工艺不同,其产品的单体同位素值也会有一定的差异。对一种国产苯乙烯标准液和一种国外产苯乙烯标准液进行氢同位素测定,国产标准的氢同位素值为-74.136‰,国外标准的氢同位素值为-65.184‰,存在比较明显的差别。
由于没有找到不含氯代物的污染场地,暂时无法进行野外实际样品的检测。 3 结论
本文建立了吹扫捕集-气相色谱/同位素质谱法测定水中苯乙烯单体氢同位素的方法,并对同位素分馏的影响因素进行了考查。本方法虽然采用25 mL的定量环,是普通5 mL定量环进样量的5倍,而且采用专用的挥发性色谱柱Rtx-624,但是方法的检出限依然较高,需要进一步提高前处理的浓缩倍数,从而降低检出限以满足野外实际样品检测的需要。
鉴于本方法采用不分流进样器检出限还有降低的空间,可在吹扫完成后进行冷阱浓缩,进一步降低检出限。
| [1] | Sano M, Yotsui Y, Abe H, et al. A new technique for the detection of metabolites labeled by the isotope 13C using mass fragmentography[J]. Biomed:Mass Spectrom, 1976, 3(1):1-3. |
| [2] | Schmidt T C, Zwank L, Elsner M, et al. Compound-specific stable isotope analysis of organic contaminants in natural environments:A critical review of the state of the art, prospects, and future challenges[J]. Anal Bioanal Chem, 2004, 378(2):283-300. |
| [3] | Meckenstock R U, Morasch B, Griebler C, et al. Stable isotope fractionation analysis as a tool to monitor biodegradation in contaminated acquifers[J]. J Contam Hydrol, 2004, 75(3-4):215-255. |
| [4] | Elsner M. Stable isotope fractionation to investigate natural transformation mechanisms of organic contaminants:Principles, prospects and limitations[J]. J Environ Monit, 2010, 12(11):2005-2031. |
| [5] | Hofstetter T B, Berg M. Assessing transformation processes of organic contaminants by compound-specific stable isotope analysis[J]. TrAC Trends Anal Chem, 2011, 30(4):618-627. |
| [6] | Aelion C M, Hohener P, Hunkeler D, et al. Environmental isotopes in bioremediation and biodegradation[M]. CRC Press, 2009. |
| [7] | Elsner M, Jochmann M A, Hofstetter T B, et al. Current challenges in compound-specific stable isotope analysis of environmental organic contaminants[J]. Analytical and bioanalytical chemistry, 2012, 403(9): 2471-2491. |
| [8] | A guide for assessing biodegradation and source identification of organic ground water contaminants using compound specific isotope analysis (CSIA)[M]. Office of Research and Development, National Risk Management Research Laboratory, US Environmental Protection Agency, 2008. |
| [8] | Hunkeler D, Meckenstock RU, Sherwood Lollar B, et al. A guide for assessing biodegradation and source identification of organic ground water contaminants using compound specific isotope analysis(CSIA) office of research and development. US EPA, Oklahoma, USA. 2008. |
| [9] | Eisenmann H, Fischer A. Isotopenuntersuchungen in der altlastenbewertung[C]//Franzius V, Altenbockum M, Gerhold T(eds) handbuch der altlastensanierung und flächenmanagement. Verlagsgruppe Hüthig Jehle Rehm, München. 2010. |
| [10] | Boyd T J, Osburn C L, Johnson K J, et al. Compound-specific isotope analysis coupled with multivariate statistics to source-apportion hydrocarbon mixtures[J]. Environ Sci Technol, 2006, 40(6):1916-1924. |
| [11] | Walker S E, Dickhut R M, Chisholm-Brause C, et al. Molecular and isotopic identification of PAH sources in a highly industrialized urban estuary[J]. Org Geochem, 2005, 36(4):619-632. |
| [12] | Hunkeler D, Chollet N, Pittet X, et al. Effect of source variability and transport processes on carbon isotope ratios of TCE and PCE in two sandy aquifers[J]. J Contam Hydrol, 2004, 74(1-4):265-282. |
| [13] | Blessing M, Schmidt T C, Dinkel R, et al. Delineation of multiple chlorinated ethene sources in an industrialized area:A forensic field study using compound-specific isotope analysis[J]. Environ Sci Technol, 2009, 43(8):2701-2707. |
| [14] | Wang Y, Huang Y, Huckins J N, et al. Compound-specific carbon and hydrogen isotope analysis of sub-parts per billion level waterborne petroleum hydrocarbons[J]. Environ Sci Technol, 2004, 38(13):3689-3697. |
| [15] | Mudge S M, Meier-Augenstein W, Eadsforth C, et al. What contribution do detergent fatty alcohols make to sewage discharges and the marine environment[J]. J Environ Monit, 2010, 12(10):1846-1856. |
| [16] | 韩 辉, 钟宁宁, 王延年, 等. 页岩气稳定碳同位素组成特征及应用前景[J]. 地质科技情报, 2014, 33(2):134-139. HAN Hui, ZHONG Ning-ning, WANG Yan-nian, et al. Characteristics of stable carbon isotope of shale gas and their applicaion prospects[J]. Geological Science and Technology Information, 2014, 33(2):134-139. |
| [17] | 郭志刚, 杨作升, 林 田, 等. 东海泥质区单体正构烷烃的碳同位素组成及物源分析[J]. 第四纪研究, 2006, 26(3):384-390. GUO Zhi-gang, YANG Zuo-sheng, LIN Tian, et al. Compound specific carbon isotope compositions of individual n-alknes in the East China sea mud areas[J]. Quaternary Sciences, 2006, 26(3):384-390. |
| [18] | 张玉龙, 冉 勇. 珠江中下游颗粒有机质的碳氮稳定同位素, 氨基酸和木质素组成及其地球化学意义[J]. 地球化学, 2014(2):114-121. ZHANG Yu-long, RAN Yong. Stable carbon and nitrogen isotopic, amino acids and lignin compositions and geochemical significance of particulate organic matter from the middle and lower reaches of the Pearl River[J]. Geochimica, 2014(2):114-121. |
| [19] | 张 杰, 贾国东. 植物正构烷烃及其单体氢同位素在古环境研究中的应用[J]. 地球科学进展, 2009, 24(8):874-881. ZHANG Jie, JIA Guo-dong. Application of pant-derived alkanes and their compound specific hydrogen isotopic composition in paleo environment research[J]. Advances in Earth Science, 2009, 24(8):874-881. |
| [20] | 贺行良, 刘昌岭, 王江涛, 等. 气相色谱-同位素比值质谱法测定天然气水合物气体单体碳氢同位素[J]. 岩矿测试, 2012, 31(1):154-158. HE Xing-liang, LIU Chang-ling, WANG Jiang-tao, et al. Measurement of carbon and hydrogen isotopes of natural gas hydrate-bound gases by gas chromatography-isotope ratio mass spectrometry[J]. Rock and Mineral Analysis, 2012, 31(1):154-158. |
 2015, Vol. 34
2015, Vol. 34