文章信息
- 吴晓荣, 张蓓蓓, 余云飞, 黄蓉, 颜明娟, 倪康, 崔静雅, 王慎强, 程谊
- WU Xiao-rong, ZHANG Bei-bei, YU Yun-fei, HUANG Rong, YAN Ming-juan, NI Kang, CUI Jing-ya, WANG Shen-qiang, CHENG Yi
- 硝化抑制剂对典型茶园土壤尿素硝化过程的影响
- Effects of nitrification inhibitors on nitrification rate of urea in four typical tea soils
- 农业环境科学学报, 2017, 36(10): 2063-2070
- Journal of Agro-Environment Science, 2017, 36(10): 2063-2070
- http://dx.doi.org/10.11654/jaes.2017-0488
文章历史
- 收稿日期: 2017-04-03
2. 江苏省农业委员会, 南京 210036;
3. 土壤与农业可持续发展国家重点实验室(中国科学院南京土壤研究所), 南京 210008;
4. 福建省农业科学院土壤肥料研究所, 福州 350013;
5. 中国农业科学院茶叶研究所, 杭州 310083;
6. 南京师范大学环境学院, 南京 210023
2. Jiangsu Agriculture Commission, Nanjing 210036, China;
3. State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, China;
4. Institute of Soil and Fertilizer, Fujian Academy of Agricultural Sciences, Fuzhou 350013, China;
5. Tea Research Institute, Chinese Academy of Agriculture Sciences, Hangzhou 310008, China;
6. School of Environment
我国是传统的茶叶种植大国,2013年我国茶叶总种植面积约176万hm2[1],约占世界茶园种植面积的59%。与其他的农作物相比,茶树独具特点。茶树是典型的喜酸好铝作物[2],生长于pH值为3.0~6.8的土壤中,但最适宜pH值为4.5~5.5[3]。随着植茶年限的增加,茶园自身物质循环和茶树根系代谢致使土壤pH值下降,钙、镁等盐基离子和微量元素缺乏,铝、氟和多酚类物质逐渐富集[4-6]。茶树喜铵厌硝,易于吸收铵态氮肥。茶树这些特性无疑决定其与其他生态系统存在不同的微生物群落结构及氮循环特征。
早在20世纪初,人们普遍认为pH < 5的土壤基本上不发生硝化作用[7]。由于茶园土壤多呈酸性,加上酸雨、人为施肥、管理不当及茶树生长过程中的自身代谢等种种原因,茶园土壤酸化日趋严重。在20世纪90年代,江苏、浙江、安徽3省的茶园土壤pH < 4.0的几率由1990年的13.7%上升到1998年的43.9%[8]。2008—2010年间江苏省21个典型茶场的调查结果显示,所调查的茶园土壤pH值均低于茶树生长最适值5.5,其中pH < 4.0的茶园占到了42.8%[9]。因此,人们一直认为酸性茶园土壤硝化速率可以忽略不计。但事实上茶园土壤尽管pH值较低,但仍存在明显的硝化作用[10-12]。韩文炎等[3]在2011年测定了我国130个茶园土壤的净硝化速率,发现土壤样本净硝化速率在0~3.00 mg·kg-1·d-1之间的占71.6%,净硝化速率超过3.00 mg·kg-1·d-1的土壤样本占18.5%,超过5.00 mg·kg-1·d-1的占5.4%,多数NO3-浓度特别高的土壤pH < 4。有研究表明硝化作用甚至可以发生在pH为2.9的极酸性茶园土壤中[13]。
目前我国茶园氮肥使用量为0~2600 kg·hm-2,主要产茶区平均为553 kg·hm-2,且有不断提高的趋势。随着施氮量的增加,茶园土壤NO3-浓度显著提高[3]。茶树是喜铵厌硝作物,硝化作用产生的硝态氮在土壤中的大量累积不利于茶树对氮的吸收,而茶树生长于热带亚热带地区,其高降雨量必然导致大量硝态氮通过径流、淋洗和反硝化损失,产生环境负效应。此外,硝化致酸,进一步加剧土壤酸化[14]。可见,控制茶园土壤硝化作用不仅有利于茶树对氮肥的吸收,还能降低氮肥的损失。目前控制土壤硝化过程较为普遍的措施是施用硝化抑制剂,常用的硝化抑制剂为双氰胺(DCD)、2-氯-6-三氯甲基吡啶(Nitrapyrin)和3,4-二甲基吡唑磷酸盐(DMPP),主要应用于水稻、小麦、玉米、蔬菜、草地等生态系统[15-18],而其在茶园这种独特生态系统中的抑制效果鲜有报道。目前,尚不明确适宜茶园酸性土壤的硝化抑制剂种类、用量及效果,因此有必要研究不同种类硝化抑制剂对茶园土壤硝化过程的影响,其研究结果将为控制茶园土壤酸化和提高氮肥利用率提供依据。
本实验以我国4种典型茶园土壤为研究对象,通过室内好气培养试验,探索尿素配施2种不同浓度的DCD和Nitrapyrin对硝化过程的抑制效果,从而筛选出针对茶园土壤有效的硝化抑制剂种类及用量。
1 材料与方法 1.1 供试土壤供试土壤采自福建安溪(植茶年限为22年,AX)、浙江杭州(植茶年限为100年,HZ)、安徽郎溪(植茶年限为60年,LX)和江苏宜兴(植茶年限为30年,YX)。其分别发育于花岗岩、石灰岩、第四纪红土和第四纪红土。同一区域的茶园每年的施肥时间和施肥量基本相同,福建茶园在每年11月施用114 kg N·hm-2复合肥;浙江茶园在每年2月和5月施用225 kg N·hm-2尿素或硫酸铵,在9—10月施用103.5 kg N·hm-2有机肥或120 kg N·hm-2复合肥;安徽茶园在每年2月中旬、5月下旬和7月上旬分别施用225、150、150 kg N·hm-2尿素,在10月底左右施用138 kg N·hm-2有机肥和112.5 kg N·hm-2复合肥;江苏茶园在每年3月初施用149 kg N·hm-2菜籽饼,在9—11月施用120 kg N·hm-2有机-无机复合肥。
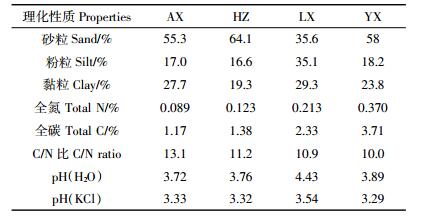
取土深度为0~20 cm,样品多点采集混合,样品中的石头、植物根系和易见的动物剔除后,在尽可能短的时间内运回实验室。过2 mm筛并将样品分为两部分,多数存放在4 ℃备用,少量风干供土壤基本理化性质测定。供试土壤的理化性质见表 1。
试验选取2种常用的硝化抑制剂,分别为双氰胺(Dicyandiamide,浓度98.00%,白色结晶性粉末,分析纯,上海国药集团生产)和2-氯-6-三氯甲基吡啶(Nitrapyrin,浓度24.00%,浅黄色乳油,浙江奥复托化工有限公司生产)。设置5个处理,每个处理3个重复:(1)尿素(CK);(2)尿素+2% DCD(2% DCD)(DCD占纯氮量的2%,下同);(3)尿素+10% DCD(10% DCD);(4)尿素+0.27% Nitrapyrin(0.27% Nitrapyrin);(5)尿素+0.54% Nitrapyrin(0.54% Nitrapyrin)。施用100 mg N·kg-1的尿素,硝化抑制剂施用量为其占施入纯氮量的百分比,各硝化抑制剂用量均为推荐用量。对于4种典型茶园土壤,称取相当于20 g干土重的新鲜土样,25 ℃预培养1 d,以调动土壤中微生物活性。预培养结束后,向三角瓶中分别加入尿素和硝化抑制剂溶液,使其尽可能均匀分布于土壤表面,同时加入蒸馏水,调节土壤含水量至60%田间持水量。用硅胶塞密封,25 ℃下恒温培养28 d,每日去塞通气20 min左右,每隔2 d补水1次,以补充因蒸发导致的水分损失。分别在添加硝化抑制剂后的2、5、9、14、21、28 d采集土壤样品,采集后的土壤样品用2 mol·L-1的KCl溶液提取,土液比为1:5,25 ℃ 250 r·min-1振荡1 h,过滤,收集滤液于塑料瓶中,置于4 ℃冰箱直至分析。
1.3 测定方法及数据分析土样中NH4+-N和NO3--N浓度使用Skalar连续流动分析仪测定。土壤pH值用KCl浸提液测定,土液比为1:5,玻璃电极法测定。所有测定均重复3次。
净硝化速率计算公式为:
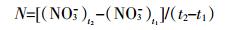 (1)
(1) 式中:N为净硝化速率,mg·kg-1·d-1;(NO3-)t2和(NO3-)t1分别是培养t2(d)和t1(d)时浓度,mg·kg-1。
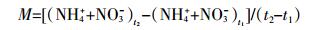 (2)
(2) 式中:M为净矿化速率,mg·kg-1·d-1;(NH4++NO3-)t2和(NH4++NO3-)t1分别是培养t2(d)和t1(d)时浓度,mg·kg-1。
硝化抑制率计算公式:
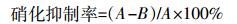 (3)
(3) 式中:A为不加抑制剂处理的土壤培养前后NO3--N浓度之差,mg·kg-1;B为添加硝化抑制剂处理的土壤培养前后NO3--N浓度之差,mg·kg-1。
1.4 数据处理采用Microsoft Excel 2007数据处理,Origin 2016作图,SPSS 20.0数据分析,处理间比较以3个重复的平均值使用邓肯新负极差法,采用全部观测值进行相关分析。图表中数据用平均值±标准差表示。
2 结果与分析 2.1 pH值变化结果表明,供试茶园土壤初始pH值在3.72~4.43之间,均低于茶园土壤最适宜pH值(4.5~5.5),土壤酸化严重(图 1中pH通过KCl浸提液测定)。所有处理土壤pH值在培养的前21 d呈逐渐上升趋势,尤其是前2 d上升最为迅速,而后缓慢下降或保持不变。整个培养期间茶园土壤的pH值增加了0.18~0.31个单位,增加最明显的为郎溪茶园土壤0.27% Nitrapyrin处理。培养期间土壤pH值上升可能是因为尿素水解生成NH4+的过程消耗H+。与对照相比,无论是加入DCD还是Nitrapyrin,均对土壤pH值无显著影响。
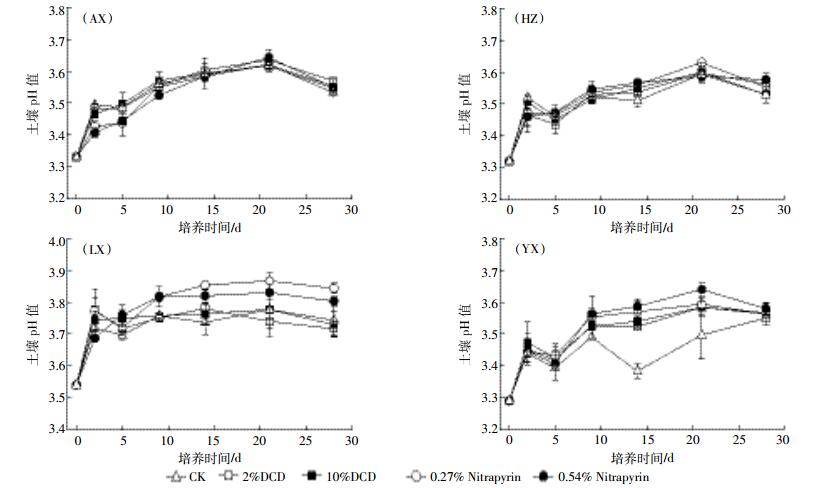
|
| 图 1 不同硝化抑制剂对茶园土壤pH值的影响 Figure 1 Soil pH from four typical tea soils treated with two different concentrations of DCD and Nitrapyrin |
培养过程中各处理的NH4+和NO3-浓度变化如图 2所示。安溪土壤对照和DCD处理的NH4+浓度先升高后保持不变,而Nitrapyrin处理的NH4+浓度先迅速增加然后缓慢上升,其在培养的前9 d低于对照和DCD处理,但在培养14 d后高于对照和DCD处理。杭州土壤在培养期间各处理的NH4+浓度均呈缓慢上升趋势。郎溪土壤对照和DCD处理的NH4+浓度先快速下降,21 d后有小幅上升,而Nitrapyrin处理的NH4+浓度逐渐上升。宜兴土壤所有处理NH4+浓度在培养期间逐渐上升。安溪土壤对照与DCD处理的NO3-浓度在培养期间逐渐上升,而Nitrapyrin处理则保持不变。杭州土壤在培养期间对照和DCD处理的NO3-浓度逐渐上升,而Nitrapyrin处理迅速下降至接近零值。郎溪土壤对照和DCD处理的NO3-浓度在培养的前21 d迅速上升而后保持不变,其变化规律恰好与NH4+相反。宜兴土壤DCD处理的NO3-浓度在培养的前14 d缓慢升高,14 d后基本没有变化,在培养期间0.27% Nitrapyrin处理的NO3-浓度保持相对稳定,而0.54% Nitrapyrin的NO3-浓度逐渐下降。
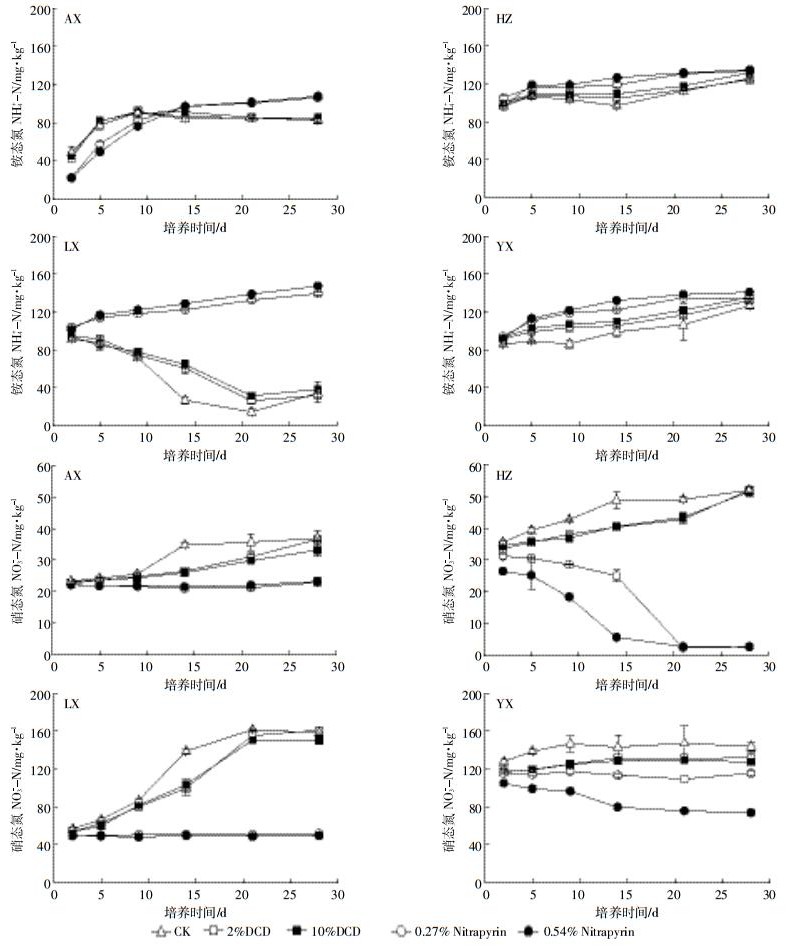
|
| 图 2 不同种类、不同剂量硝化抑制剂对茶园土壤铵态氮、硝态氮的影响 Figure 2 Changes of soil ammonium and nitrate concentration in four typical tea soils treated with two different concentrations of DCD and Nitrapyrin |
硝化抑制率是表征硝化抑制剂对土壤硝化过程抑制强度的一个重要指标,其值越高表明硝化抑制剂对土壤硝化过程抑制强度越强。据表 2可知,对于4种供试茶园土壤,DCD在培养过程中对硝化过程的抑制效果随培养时间的延长呈下降趋势,且高浓度DCD仅在培养第28 d时才表现出比低浓度DCD更强的抑制效果。培养第28 d后,在安溪和宜兴茶园土壤中DCD的抑制率仍可达12.15%~59.68%,而对于杭州和郎溪茶园土壤,无论DCD浓度高低,培养第28 d后抑制效果消失。对于所研究的4种茶园土壤,DCD处理在培养期间的抑制效果表现为宜兴土壤>安溪土壤>杭州土壤>郎溪土壤。与DCD相比,无论加入Nitrapyrin浓度高低,在培养期间其对4种茶园土壤的硝化抑制率接近甚至超过100%,表明Nitrapyrin几乎能完全抑制茶园土壤硝化作用,且抑制有效期超过28 d。
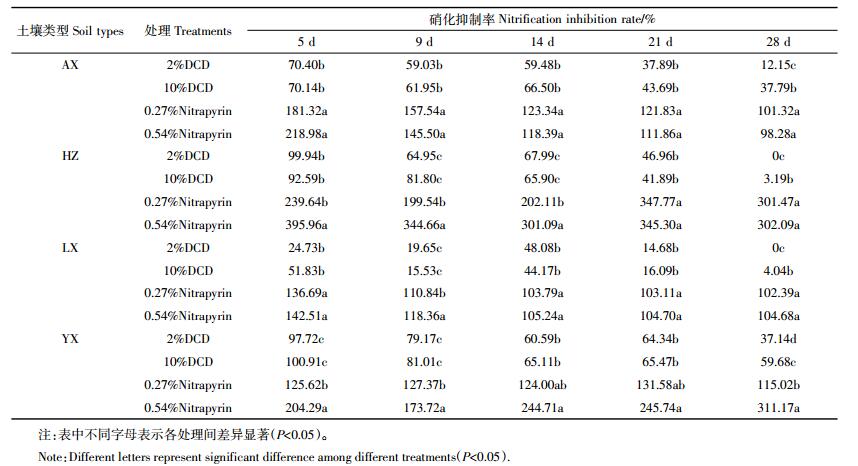
|
如图 3所示,对于供试4种茶园土壤,2% DCD和10% DCD与对照相比对净硝化速率无显著影响,而0.27% Nitrapyrin和0.54% Nitrapyrin则显著降低了净硝化速率。加入DCD的茶园土壤与对照相比,对土壤净矿化速率没有显著影响,但郎溪土壤2% DCD加入显著提高了净矿化速率(P < 0.05)。安溪土壤0.27% Nitrapyrin和0.54% Nitrapyrin处理净矿化速率分别是对照处理的1.79、1.83倍。与对照处理相比,杭州和宜兴土壤Nitrapyrin加入显著抑制净矿化速率,且杭州土壤低浓度的抑制效果强而宜兴土壤则相反。郎溪土壤0.27% Nitrapyrin处理的净矿化速率与对照处理相比无显著差异,而0.54% Nitrapyrin加入则可显著提高净矿化速率。
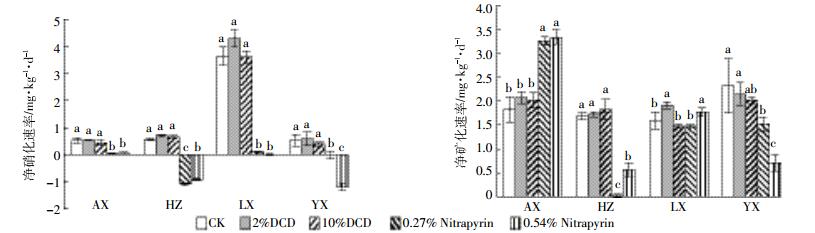
|
| 图中不同小写字母表示各处理间差异显著(P < 0.05) Different lowercase letters indicate significant differences (P < 0.05) among different treatments 图 3 硝化抑制剂对茶园土壤28 d的净硝化速率和净矿化速率的影响 Figure 3 Nitrification and mineralization rates in four typical tea soils treated with two different concentrations of DCD and Nitrapyrin |
传统观点认为土壤pH < 5.0时硝化作用较慢,甚至缺失[7, 19]。本研究选取的茶园土壤pH值均小于4.43(表 1),但其净硝化速率并不低,在0.52~3.65 mg·kg-1·d-1之间,尤以郎溪茶园土壤硝化速率最高。韩文炎等[3]测定了我国130个茶园土壤的净硝化速率,发现土壤净硝化速率在-6.08~6.54 mg·kg-1·d-1之间,平均为1.62 mg·kg-1·d-1。可见,尽管茶园土壤pH值很低,但是硝化速率并不低。本研究结果表明,加入尿素后4种茶园土壤发生显著的硝化作用,但尿素和Nitrapyrin混施处理中,NO3-浓度在培养期间却没有增加。这表明尿素施入后,茶园土壤发生了氨氧化过程(自养硝化),且Nitrapyrin完全抑制了培养期间硝态氮产生。由于硝化抑制剂抑制的是氨氧化过程,可以推断茶园土壤硝态氮主要来自于自养硝化而非异养硝化过程[20-22]。这与人们通常认为的pH值较低土壤中硝态氮主要来自异养硝化过程的观点相左[23-26]。可能的原因是茶园土壤存在耐酸性或者嗜酸性的自养硝化细菌[13, 27],而且还存在大量氨氧化古菌[28]。茶园一般施入铵态氮肥,由于铵态氮是氨氧化过程的底物,其施入必然刺激氨氧化细菌和古菌的生长,进而提高自养硝化速率,尤其尿素水解能短暂提高土壤pH值,进一步提高自养硝化速率。茶树喜铵,硝化不利于茶树对氮的吸收,且硝化致酸,会进一步酸化土壤,当土壤pH值低于其最适宜pH值的下限,茶树则无法生长。茶树生长在热带和亚热带多雨地区[29],硝化产生的硝态氮易随径流和淋洗而损失。因此,选用硝化抑制剂抑制自养硝化是提高茶园土壤氮肥利用率以及降低氮损失的关键手段。
3.2 2种硝化抑制剂的硝化抑制效果比较本研究表明,Nitrapyrin和DCD均能抑制茶园土壤自养硝化过程,但Nitrapyrin(98.28%~100%)比DCD(0~59.68%)抑制效果好,与他人在茶园酸性土壤和石灰性土壤中的研究结果一致[30-31]。DCD在土壤中有效抑制期与DCD的降解周期有关[32],同时DCD施用效果也受温度的影响,其在5、15、25 ℃下有效抑制期分别为89、37、18 d[33]。徐星凯等[34]使用同位素示踪技术,通过小麦盆栽试验发现DCD有效抑制硝化作用达2个月。本研究中DCD处理在培养期间的抑制效果表现为宜兴土壤>安溪土壤>杭州土壤>郎溪土壤。研究表明,硝化抑制剂的施用效果受土壤有机质含量和质地的影响[15, 33, 35-36]。在土壤中施用硝化抑制剂,NO-2的生成量与砂粒含量呈负相关[37]。在砂土、壤土和黏土中,加入DCD后硝化抑制率分别为96.5%~99.4%、49.3%~79.4%和66.9%~85.6%,其施用效果总体表现为砂土>黏土>壤土[38]。本研究中安溪、杭州和宜兴土壤属于砂土,而郎溪土壤属于黏壤土,这就解释了为什么DCD在郎溪茶园土壤上的抑制效果较差。
施用Nitrapyrin几乎能够100%抑制茶园土壤的自养硝化速率,表明Nitrapyrin的抑制效果非常强劲,适合于控制茶园土壤的硝化作用。Nitrapyrin在石灰性砂土中的抑制率在96.6%~99.3%之间[31]。本研究表明,Nitrapyrin不仅抑制了硝化过程,在杭州和宜兴土壤培养期间甚至出现NO3-显著下降,说明Nitrapyrin施入刺激了杭州和宜兴土壤NO3-消耗。而好氧条件下NO3-的消耗主要以硝态氮同化和反硝化过程为主[39],一般情况下只要有铵态氮存在,硝态氮同化很难进行[40],即使Nitrapyrin含有大量C源,在大量的铵态氮存在下,应先促进铵态氮同化,直到铵态氮被消耗完,才会促进硝态氮同化。因此,杭州和宜兴土壤中加入Nitrapyrin硝态氮随培养时间下降可能是反硝化造成的,其原因可能是Nitrapyrin及其溶剂中含有易分解的C源,直接为反硝化细菌提供能量和电子而促进反硝化[41-42]。Notton等[43]研究也发现,Nitrapyrin在植物残根及丙酮等C源的存在下,抑制硝化过程的同时刺激了砂土的硝酸盐还原。
4 结论本研究表明,在28 d的培养期间,Nitrapyrin几乎可以完全抑制茶园土壤硝化作用,而DCD的抑制效果相对较差,抑制率仅为0~60%。Nitrapyrin对于硝化过程的完全抑制表明硝态氮产生可能主要来自于自养硝化。因此,Nitrapyrin是一种针对茶园土壤比较高效的硝化抑制剂,配施铵态氮肥可以有效降低土壤硝酸盐累积。但是,以上结果均是基于实验室培养试验的结果,将来需要通过田间试验进一步验证本研究结果的可靠性。
| [1] |
朱永兴. 最近5年世界各主要产茶国茶园面积和茶叶年产量[J]. 茶叶科学, 2015, 35(4): 12. ZHONG Yong-xing. Plantation area and annual output of tea in the world over the past 5 years[J]. Journal of Tea Science, 2015, 35(4): 12. |
| [2] |
Fung K F, Carr H P, Zhang J H, et al. Growth and nutrient uptake of tea under different aluminium concentrations[J]. Journal of the Science of Food and Agriculture, 2008, 88(9): 1582-1591. DOI:10.1002/(ISSN)1097-0010 |
| [3] |
韩文炎, 徐建明. 茶园土壤NO3--N含量与净硝化速率的研究[J]. 茶叶科学, 2011, 31(6): 513-520. HAB Wen-yan, XU Jian-ming. NO3--N concentration and net nitrification rate in tea soils[J]. Journal of Tea Science, 2011, 31(6): 513-520. |
| [4] |
丁瑞兴, 黄骁. 茶园-土壤系统铝和氟的生物地球化学循环及其对土壤酸化的影响[J]. 土壤学报, 1991, 28(3): 229-236. DING Rui-xing, HUANG Xiao. Biogeochemical cycle of aluminum and fluorine in tea garden soil system and its relationship to soil acidification[J]. Acta Pedologica Sinica, 1991, 28(3): 229-236. |
| [5] |
Han W Y, Kemmitt S J, Brookes P C. Soil microbial biomass and activity in Chinese tea plantations of varying stand and productivity[J]. Soil Biology and Biochemistry, 2007, 39(7): 1468-1478. DOI:10.1016/j.soilbio.2006.12.029 |
| [6] |
Pansombat K, Kanazawa S, Horiguchi T. Microbial ecology in tea soils:Ⅰ. Soil properties and microbial populations[J]. Soil Science and Plant Nutrition, 1997, 43(2): 317-327. DOI:10.1080/00380768.1997.10414756 |
| [7] |
Noyes H A, Conner S D. Nitrates, nitrification, and bacterial contents of five typical acid soils as affected by lime, fertilizer, crops and moisture[J]. J Agric Res, 1919, 16: 27-60. |
| [8] |
马立锋, 石元值, 阮建云. 苏、浙、皖茶区茶园土壤pH状况及近十年来的变化[J]. 土壤通报, 2000, 31(5): 205-207. MA Li-feng, SHI Yuan-zhi, RUAN Jian-yun. Soil pHs in the tea gardens in Jiangsu, Zhejiang, and Anhui Provinces and changes of soil pH in the past decade[J]. Chinese Journal of Soil Science, 2000, 31(5): 205-207. |
| [9] |
张倩, 宗良纲, 曹丹, 等. 江苏省典型茶园土壤酸化趋势及其制约因素研究[J]. 土壤, 2011, 43(5): 751-757. ZHANG Qian, ZONG Liang-gang, CAO Dan, et al. Study on soil acidification and its restrictive factors of typical tea garden in Jiangsu Province[J]. Soils, 2011, 43(5): 751-757. |
| [10] |
Xue D, Yao H Y, Huang C Y. Microbial biomass, N mineralization and nitrification, enzyme activities, and microbial community diversity in tea orchard soils[J]. Plant and Soil, 2006, 288(1/2): 319-331. |
| [11] |
Zhu T B, Zhang J B, Meng T Z, et al. Tea plantation destroys soil retention of NO3- and increases N2O emissions in subtropical China[J]. Soil Biology and Biochemistry, 2014, 73: 106-114. DOI:10.1016/j.soilbio.2014.02.016 |
| [12] |
Hayatsu M, Kosuge N. Effects of difference in fertilization treatments on nitrification activity in tea soils[J]. Soil Science and Plant Nutrition, 1993, 39(2): 373-378. DOI:10.1080/00380768.1993.10417010 |
| [13] |
Hayatsu M. The lowest limit of pH for nitrification in tea soil and isolation of an acidophilic ammonia oxidizing bacterium[J]. Soil Science and Plant Nutrition, 1993, 39(2): 219-226. DOI:10.1080/00380768.1993.10416993 |
| [14] |
Helyar K R. Nitrogen cycling and soil acidification[J]. J Aust Inst Agric Sci, 1976, 42(4): 217. |
| [15] |
孙志梅, 武志杰, 陈利军, 等. 硝化抑制剂的施用效果、影响因素及其评价[J]. 应用生态学报, 2008, 19(7): 1611-1618. SUN Zhi-mei, WU Zhi-jie, CHEN Li-jun, et al. Application effect, affecting factors, and evaluation of nitrification inhibitor:A review[J]. Chinese Journal of Applied Ecology, 2008, 19(7): 1611-1618. |
| [16] |
Qiao C L, Liu L I, Hu S J, et al. How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input[J]. Global Change Biology, 2015, 21(3): 1249-1257. DOI:10.1111/gcb.2015.21.issue-3 |
| [17] |
Gilsanz C, Báez D, Misselbrook T H, et al. Development of emission factors and efficiency of two nitrification inhibitors, DCD and DMPP[J]. Agriculture, Ecosystems and Environment, 2016, 216: 1-8. DOI:10.1016/j.agee.2015.09.030 |
| [18] |
Reiner R, Rudolf S. The effect of nitrification inhibitors on the nitrous oxide(N2O) release from agricultural soils:A review[J]. Journal of Plant Nutrition and Soil Science, 2015, 178(2): 171-188. DOI:10.1002/jpln.v178.2 |
| [19] |
Weber D F, Gainey P L. Relative sensitivity of nitrifying organisms to hydrogen ions in soils and solutions[J]. Soil Science, 1962, 94(3): 138-145. DOI:10.1097/00010694-196209000-00002 |
| [20] |
Boer W D, Duyts H, Laanbroek H J. Autotrophic nitrification in a fertilized acid heath soil[J]. Soil Biology and Biochemistry, 1988, 20(6): 845-850. DOI:10.1016/0038-0717(88)90091-0 |
| [21] |
Hayatsu M, Kosuge N. Autotrophic nitrification in acid tea soils[J]. Soil Science and Plant Nutrition, 1993, 39(2): 209-217. DOI:10.1080/00380768.1993.10416992 |
| [22] |
Bauhus J, Meyer A C, Brumme R. Effect of the inhibitors nitrapyrin and sodium chlorate on nitrification and N2O formation in an acid forest soil[J]. Biology and Fertility of Soils, 1996, 22(4): 318-325. DOI:10.1007/BF00334576 |
| [23] |
Kreitinger J P, Klein T M, Novick N J, et al. Nitrification and characteristics of nitrifying microorganisms in an acid forest soil[J]. Soil Science Society of America Journal, 1985, 49(6): 1407-1410. DOI:10.2136/sssaj1985.03615995004900060015x |
| [24] |
Wood P M. Autotrophic and heterotrophic mechanisms for ammonia oxidation[J]. Soil Use and Management, 1990, 6(2): 78-79. DOI:10.1111/sum.1990.6.issue-2 |
| [25] |
Zhang J B, Müller C, Zhu T B, et al. Heterotrophic nitrification is the predominant NO3- production mechanism in coniferous but not broad-leaf acid forest soil in subtropical China[J]. Biology and Fertility of Soils, 2011, 47(5): 533-542. DOI:10.1007/s00374-011-0567-z |
| [26] |
Zhang J B, Sun W J, Zhong W H, et al. The substrate is an important factor in controlling the significance of heterotrophic nitrification in acidic forest soils[J]. Soil Biology and Biochemistry, 2014, 76: 143-148. DOI:10.1016/j.soilbio.2014.05.001 |
| [27] |
Walker N, Wickramasinghe K N. Nitrification and autotrophic nitrifying bacteria in acid tea soils[J]. Soil Biology and Biochemistry, 1979, 11(3): 231-236. DOI:10.1016/0038-0717(79)90067-1 |
| [28] |
Yao H Y, Gao Y M, Nicol G W, et al. Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils[J]. Applied and Environmental Microbiology, 2011, 77(13): 4618-4625. DOI:10.1128/AEM.00136-11 |
| [29] |
Li S Y, Wu X, Xue H, et al. Quantifying carbon storage for tea plantations in China[J]. Agriculture, Ecosystems and Environment, 2011, 141(3): 390-398. |
| [30] |
Wickramasinghe K N, Rodgers G A, Jenkinson D S. Nitrification in acid tea soils and a neutral grassland soil:Effects of nitrification inhibitors and inorganic salts[J]. Soil Biology and Biochemistry, 1985, 17(2): 249-252. DOI:10.1016/0038-0717(85)90123-3 |
| [31] |
刘涛, 梁永超, 褚贵新, 等. 三种硝化抑制剂在石灰性土壤中的应用效果比较[J]. 土壤, 2011, 43(5): 758-762. LIU Tao, LIANG Yong-chao, CHU Gui-xin, et al. Effect comparison of three different types of nitrification inhibitors(DCD, DMPP and Nitrapyrin) in calcareous soils[J]. Soils, 2011, 43(5): 758-762. |
| [32] |
Hauser M, Haselwandter K. Degradation of dicyandiamide by soil bacteria[J]. Soil Biology & Biochemistry, 1990, 22(1): 113-114. |
| [33] |
McGeough K L, Watson C J, Müller C, et al. Evidence that the efficacy of the nitrification inhibitor dicyandiamide(DCD) is affected by soil properties in UK soils[J]. Soil Biology and Biochemistry, 2016, 94: 222-232. DOI:10.1016/j.soilbio.2015.11.017 |
| [34] |
徐星凯, 周礼恺.脲酶抑制剂/硝化抑制剂对土壤中尿素氮转化及形态分布的影响[J].土壤学报, 2000, 37(3):339-345. XU Xing-kai, ZHOU Li-kai, Cleemput O V. Effect of urease/nitrification inhibitors on the distribution of transformed urea-N form in soil[J]. Acta Pedologica Sinica, 2000, 37(3):339-345. http://www.cnki.com.cn/Article/CJFDTOTAL-TRXB200003006.htm |
| [35] |
Slangen J H G, Kerkhoff P. Nitrification inhibitors in agriculture and horticulture:A literature review[J]. Fertilizer Research, 1984, 5(1): 1-76. DOI:10.1007/BF01049492 |
| [36] |
Tate K R. Soil phosphorus[M]. Springer Netherlands, 1985, 329-377.
|
| [37] |
Barth G, von Tucher S, Schmidhalter U. Influence of soil parameters on the effect of 3, 4-dimethylpyrazole-phosphate as a nitrification inhibitor[J]. Biol Fertil Soils, 2001, 34: 98-102. DOI:10.1007/s003740100382 |
| [38] |
刘倩, 褚贵新, 刘涛, 等. DCD在不同质地土壤上的硝化抑制效果和剂量效应研究[J]. 中国生态农业学报, 2011, 19(4): 765-770. LIU Qian, CHU Gui-xin, LIU Tao, et al. Nitrification inhibition and dose-dependent effect of dicyandiamide on sandy, loamy and clayey soils[J]. Chinese Journal of Eco-Agriculture, 2011, 19(4): 765-770. |
| [39] |
周立祥, 黄峰源, 王世梅. 好氧反硝化菌的分离及其在土壤氮素转化过程中的作用[J]. 土壤学报, 2006, 43(3): 430-435. ZHOU Li-xiang, HUANG Feng-yuan, WANG Shi-mei. Isolation of aerobic denitrifiers and their roles in soil nitrogen transformation[J]. Acta Pedologica Sinica, 2006, 43(3): 430-435. DOI:10.11766/trxb200503090311 |
| [40] |
Jansson, Sven L. Tracer studies on nitrogen transformations in soil with special attention to mineralization-immobilization relationships[J]. Ann Roy Agric Coll Sweden, 1958, 24: 101-361. |
| [41] |
Klemedtsson L, Svensson B H, Rosswall T. A method of selective inhibition to distinguish between nitrification and denitrification as sources of nitrous oxide in soil[J]. Biology and Fertility of Soils, 1988, 6(2): 112-119. |
| [42] |
孙志梅, 武志杰, 陈利军, 等. 土壤硝化作用的抑制剂调控及其机理[J]. 应用生态学报, 2008, 19(6): 1389-1395. SUN Zhi-mei, WU Zhi-jie, CHEN Li-jun, et al. Regulation of soil nitrification with nitrification inhibitors and related mechanisms[J]. Chinese Journal of Applied Ecology, 2008, 19(6): 1389-1395. |
| [43] |
Notton B A, Watson E F, Hewitt E J. Effects of N-serve(2-chloro-6-(trichloromethyl) pyridine) formulations on nitrification and on loss of nitrate in sand culture experiments[J]. Plant and Soil, 1979, 51(1): 1-12. DOI:10.1007/BF02205921 |
 2017, Vol. 36
2017, Vol. 36