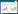文章信息
- 孙翼飞, 沈菊培, 张翠景, 孙书存, 贺纪正
- SUN Yi-fei, SHEN Ju-pei, ZHANG Cui-jing, SUN Shu-cun, HE Ji-zheng
- 模拟水位下降与刈割对高寒湿地土壤氨氧化与反硝化微生物的影响
- Effects of water table lowering and mowing on soil ammonia oxidizers and denitrifiers in alpine wetlands
- 农业环境科学学报, 2017, 36(11): 2356-2364
- Journal of Agro-Environment Science, 2017, 36(11): 2356-2364
- http://dx.doi.org/10.11654/jaes.2017-0589
文章历史
- 收稿日期: 2017-04-22
- 接受日期: 2017-07-05
2. 中国科学院大学, 北京 100049;
3. 南京大学生命科学学院, 南京 210023
2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. School of Life Sciences, Nanjing University, Nanjing 210023, China
湿地土壤是温室气体重要的源和汇,湿地生态系统在全球变化中发挥着重要作用[1-2]。氮素循环是生物地球化学循环最基础和最重要的过程,其中关键的两个步骤硝化作用和反硝化作用均由微生物所驱动[3-4]。氨氧化作用是硝化作用的限速步骤,主要由氨氧化古菌(Ammonia-oxidizing archaea,AOA)和氨氧化细菌(Ammonia-oxidizing bacteria,AOB)所驱动[5]。氨单加氧酶基因(amoA)常被用作分子标记来研究生态系统中的氨氧化微生物[6]。反硝化作用是反硝化微生物在厌氧条件下通过一系列氮氧化合物将硝酸盐还原为氮气的过程,其中亚硝酸盐还原为一氧化氮的过程是反硝化作用有别于其他硝酸盐代谢的标志性反应,是反硝化过程中的重要限速步骤,其相应的亚硝酸盐还原酶基因nirK和nirS丰度常被用来表征和预测N2O的释放[7-8]。硝化-反硝化的耦合过程是土壤氮素损失最主要的途径,尤其在含水量较高的土壤中[9]。作为我国典型的泥炭湿地,若尔盖湿地是世界上最大的高山泥炭地,面积达4605 km2 [10]。由于人类活动的影响,如人工排水、土地管理方式的改变等,使得大面积的泥炭土壤转变为草地或沙地,对土壤性质、地上植物群落组成和植被覆盖率产生了很大的影响[11]。湿地土壤类型的改变也会加快土壤有机质矿化,进而导致湿地土壤氮素流失[12]和温室气体N2O的排放,继而增加全球气候变化的风险。因此,研究地下水位及土地利用变化对硝化与反硝化过程及其参与的关键功能微生物的影响,对预测生态系统氮循环对全球气候变化的响应具有重要意义。
关于外界环境变化对若尔盖湿地土壤氮素的影响已开展了一些研究。例如:李丽等[13]研究表明泥炭地排水、地下水位降低是土壤氮素流失的重要原因;赵宁等[14]发现轻度放牧显著增加了土壤硝化速率和净矿化速率。土壤微生物丰度与群落结构对湿地水位下降、土壤养分含量变化响应较为敏感[15-16]。有研究表明,表层土壤中古菌的丰度随水位下降而下降[15],而水位变化和刈割通过影响土壤中氧气的扩散速率和氧化还原电位以及减少土壤碳源的输入影响土壤产甲烷菌和甲烷氧化菌的生长。关于功能微生物对水位下降的响应大多集中在与碳循环相关的产甲烷菌和甲烷氧化菌[17-18]。然而,关于参与氮循环的功能微生物对环境因子的响应机制尚不明确。有研究表明若尔盖湿地AOA多样性指数较低,且与土壤铵态氮和硝态氮含量显著相关[19];另有研究表明土壤水分可能是影响氨氧化和反硝化微生物群落的重要因素[20]。为探究湿地土壤氮循环功能微生物对环境干扰的响应机制,以青藏高原东部若尔盖湿地南部的泥炭湿地为试验样地,以在样地周边挖掘不同深度的排水沟来模拟水位下降,用刈割模拟土地管理方式的变化,研究人类活动干扰对土壤理化性质、微生物活性、氨氧化(AOA-amoA和AOB-amoA)和反硝化(nirK和nirS)功能微生物丰度的影响。
1 材料与方法 1.1 实验样地与设计试验样地位于四川省阿坝州红原县青藏高原东部若尔盖湿地南部的高原,坐标102°33′E、32°48′N,海拔3561 m。本研究区域分布在若尔盖高寒地区季节性积水区的高寒草甸湿地[21]。该地区泥炭层厚度在0.3~10 m之间,干物质积累平均速率为0.03 g·m-2·a-1 [22]。试验前土壤基本理化性质如下:土壤pH值在6.6~7.0之间[23],总碳(Total carbon,TC)平均含量为58.6 mg·L-1,总氮(Total nitrogen,TN)及可溶性有机碳(Dissolved organic carbon,DOC)含量分别为1.4、25.7 mg·L-1 [24]。研究样地植被类型属于沼泽草甸,优势植物种包括发草、穗三毛、鹅绒萎陵菜、高山嵩草等。土壤为泥炭土,年均温为1.1 ℃,年均降水量为752 mm,80%以上降水集中在5—9月[25]。
为评估水位下降和刈割对土壤化学性质和土壤微生物的影响,我们采用了配对裂区设计,通过在样地周围挖掘不同深度的排水沟,来模拟土壤地下水位下降,其宽度为0.5 m,共设计2个梯度,分别为20 cm(样地周围挖掘20 cm排水沟)及50 cm(样地周围挖掘50 cm排水沟),用于模拟浅水位(S)和深水位(H)。样地排水沟与一条240 m长、0.5 m宽、1 m深的主排水沟相连,排水样地随机分布在距主排水沟两侧30 m处;以不挖排水沟的样地作为对照,且随机分布在两侧排水样地外30 m处,相邻样地间隔30 m,每个设计6个重复,共18个6 m×6 m的区组;每个区组有2个2 m×2 m的亚区,分别为刈割(M)与对照处理,每个亚区间隔3 m且距样地边缘1 m。因此,本样地共6个处理,即正常水位下不刈割(CK)及刈割处理(M),浅水位不刈割(S),浅水位刈割(SM),深水位不刈割(H)及深水位刈割(HM)处理,每个处理6个重复,共36个亚区。同时,每个亚区通过刈割保持8 cm植物群落,以不刈割作为对照。样地具体设置如图 1,该模拟试验开始于2013年5月。
|
| 图 1 试验样地设置示意图 Figure 1 Experimental site design |
土壤样品采集于2014年7月,每个亚区用直径5 cm的土钻随机取3个样品混合,采集0~20 cm土壤样品。所有的样品均在3 d内运到实验室。土壤样品通过2 mm筛去除草根和石块,一部分新鲜土壤储存在4 ℃用于分析基本理化性质,另一部分经冷冻干燥后存储在-80 ℃用于分子生物学指标分析。
1.2 土壤理化性质和微生物活性测定在105 ℃烘箱中烘干12 h来测量土壤含水量(Soil moisture,SM)。采用pH计,以土水比为1:2.5(g:mL)来测定土壤pH值(Delta 320,Mettler-Toledo Instruments Co.,Shanghai,China)。土壤铵态氮(NH4+-N)和硝态氮(NO3--N)用1 mol·L-1氯化钾提取(土壤:水=1 g:5 mL),用连续流动分析仪(SAN++,Skalar,Holand)测定浓度。
硝化潜势(Potential nitrification rate,PNR)的测量使用氯酸盐抑制方法:将5.0 g新鲜土壤加入到50 mL离心管中,并向其中加入20 mL磷酸缓冲溶液(PBS)(NaCl 8 g·L-1,KCl 0.2 g·L-1,Na2HPO4 1.44 g·L-1,KH2PO4 0.24 g·L-1,pH 7.4),内含2.64 mg 1 mmol·L-1(NH4)2SO4和0.024 5 mg 50 mg·L-1 KClO3来抑制亚硝酸盐(NO2-)氧化为硝酸盐(NO3-)。土样于25 ℃条件下,在摇床中以180 r·min-1的速度培养24 h后用5 mL 2 mol·L-1氯化钾提取亚硝酸盐(NO2-),含有NO2-的上清液以N-(1-萘胺)乙二胺盐酸盐显色,用分光光度法在530 nm波长下测定浓度[26]。
土壤异养呼吸(Heterotrophic respiration,Rh)的测定方法如下:将10 g新鲜土壤加入到120 mL血清瓶中,恒温培养箱25 ℃条件下培养24 h,收集二氧化碳气体(CO2)并用气相色谱仪(Agilent 7890A GC System)测定浓度[27]。
1.3 土壤DNA提取和实时定量PCR测定称取0.25 g冻土,按照PowerSoilTM Total DNA Isolation试剂盒(Mo Bio Laboratories,Inc.,San Diego,CA,USA)提供的方法提取土壤总DNA。采用NanoDrop分光光度计(NanoDrop Technologies,USA)测定DNA纯度和浓度。将DNA样品稀释10倍后用于分子实验的模板,并存储于-20 ℃。采用iCycler iQ5仪器(Bio-Rad Laboratories,Inc.,USA)进行AOA-amoA、AOB-amoA、nirK和nirS基因的定量PCR实验,使用的引物和反应条件在表 1中列出。
功能基因的定量测定在iCycler iQ5仪器(Bio-Rad Laboratories,Inc.,USA)上完成,用SYBR GREEN作为荧光标记,反应体系为25 μL,包含12.5 μL 2 x SYBR Premix Ex TaqTM(Takara Biotechnology,Japan),每个引物0.5 μL(10 μmol·L-1)和2 μL的DNA模板(1~10 ng),其他用灭菌水补足。使用10倍连续稀释的质粒作为模板绘制标准曲线,所有基因的扩增效率在85%~100%之间,R2为0.99。利用溶解曲线和琼脂糖凝胶电泳检测扩增产物的特异性。
1.4 统计分析R 3.3.2及SPSS19软件被用来进行统计分析,当P<0.05时,差异显著。使用重复试验的双因素方差分析(Duncan检验)评估处理(模拟水位下降、刈割及二者交互作用)对土壤化学性质、微生物活性及功能微生物丰度的影响。采用Spearman相关分析来揭示土壤化学性质与土壤微生物活性及功能基因丰度间的相关性。响应比率(Response ratio)用来分析水位下降与刈割对土壤微生物的影响,在95%的置信区间下,平均值与零重叠表示处理对土壤微生物无显著影响,平均值不与零重叠表示处理对土壤微生物影响显著[32]。
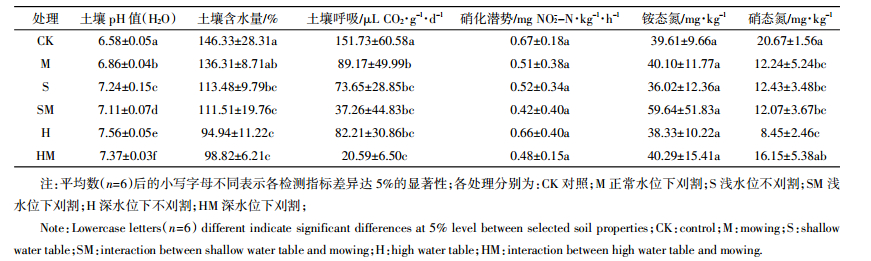
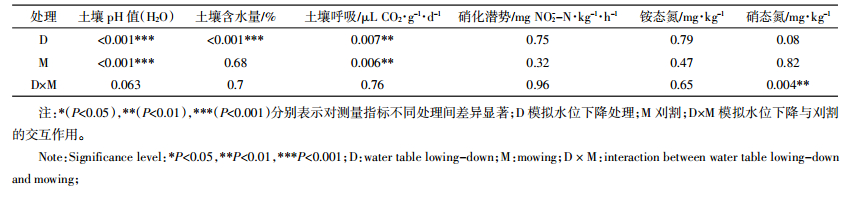
2 结果与分析 2.1 模拟水位下降与刈割对土壤化学性质和微生物活性的影响模拟水位下降与刈割对土壤化学性质和微生物活性的影响见表 2和表 3。不论是否刈割,土壤含水量随地下水位下降显著降低,土壤含水量在对照处理(CK)中最高,在深水位处理中最低。水位下降显著增加了土壤pH值,且同一水位下刈割处理的土壤pH值均显著低于对照组;水位下降与刈割对土壤硝态氮含量有显著的交互作用,硝态氮含量在对照处理中最高,在深水位处理中最低。水位下降与刈割均显著影响了土壤呼吸,土壤呼吸在对照处理中最高,深水位与刈割二者交互作用处理中最低。水位下降和刈割对土壤硝化潜势和铵态氮均没有显著影响。Spearman相关分析发现:土壤含水量与土壤呼吸显著正相关(ρ=0.549,P=0.004)。
|
|
模拟水位下降与刈割对土壤氨氧化(AOA-amoA、AOB-amoA)和反硝化微生物(nirK、nirS)功能基因丰度的影响见图 2。总体上讲,AOA-amoA基因丰度高于AOB-amoA基因丰度,nirS基因丰度高于nirK基因丰度,且各基因丰度在处理间无显著差异。AOA-amoA基因丰度范围为每克干土(0.43~1.05)×106拷贝数,AOB-amoA基因丰度范围为(0.11~1.33)×105拷贝数,nirK基因丰度范围为(0.77~4.44)×105拷贝数,nirS基因丰度范围为(1.36~5.74)×108拷贝数。刈割、水位下降与刈割二者交互作用对AOA-amoA与AOB-amoA基因丰度比有显著影响(P=0.016;P=0.045)(图 2e),对nirK与nirS基因丰度比、氨氧化与反硝化微生物丰度比无显著影响。

|
| 不同字母表示同一测量指标不同处理间差异显著(Duncan检验),未用字母标明即差异不显著 Different letters indicate significantly different values at P < 0.05(Duncan test). No letter indicante non-significant 图 2 模拟水位下降与刈割对各基因丰度的影响 Figure 2 Effects of mowing on abundances of functional genes across different treatments |
进一步分析各基因相对丰度对水位下降及刈割的响应敏感度,发现刈割显著增加了AOB-amoA基因相对丰度(图 3a)。Spearman相关分析发现,AOA-amoA与AOB-amoA基因丰度比与铵态氮含量显著负相关(ρ=-0.604,P<0.001),与土壤含水量(ρ=0.406,P=0.044)、硝态氮含量(ρ=0.531,P=0.006)显著正相关,nirS基因丰度与硝态氮含量显著负相关(ρ=-0.403,P=0.046)。
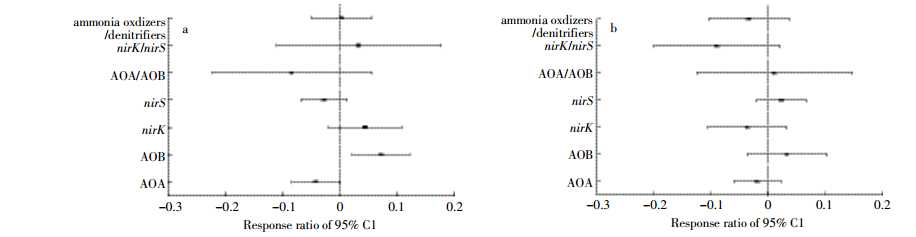
|
| AOA:AOA-amoA基因丰度,AOB:AOB-amoA基因丰度,nirK:nirK基因丰度,nirS:nirS基因丰度,AOA/AOB:AOA-amoA与AOB-amoA基因丰度比,nirK/nirS:nirK与nirS基因丰度比,ammonia oxidizers/denitrifiers:AOA-amoA与AOB-amoA基因丰度之和与nirK与nirS基因丰度之和的比值。 AOA:the abundance of AOA-amoA gene, AOB:relative abundance of AOB-amoA gene, nirK:relative abundance of nirK gene, nirS:relative abundance of nirS gene, AOA/AOB:ratio of the abundance of AOA-amoA and AOB-amoA genes, nirK/nirS:ratio of the abundance of nirK and nirS genes, ammonia oxidizers/denitrifiers:ratio of the sum of AOA-amoA and AOB-amoA genes to the sum of nirK and nirS genes. 图 3 氨氧化与反硝化基因丰度对刈割(a)与模拟水位下降(b)的响应 Figure 3 Response ratio(RR)of relative abundance of functional genes to mowing(a)and water table lowing-dwon(b) |
异养呼吸是指土壤微生物分解有机质释放CO2的过程[33],是泥炭湿地有机碳储备降低的影响因素之一,主要与土壤水分和有机质含量显著相关[34]。土壤异养呼吸主要由于土壤有机质和植物残留物的降解,与自养呼吸相比具有更高的水分敏感性[35]。本研究发现模拟水位下降与刈割均显著降低了土壤异养呼吸,主要与土壤中可溶性有机碳的降低有关。土壤呼吸与含水量显著正相关,土壤水分的降低通过影响可溶性有机碳的有效性间接影响土壤呼吸[36-37]。Wu等[11]经过为期3年的碳动态观测,发现水位下降显著促进了土壤可溶性有机碳分解,进而导致该样地0~20 cm土壤有机碳含量下降了18.5%。除此之外,土壤温度也是影响土壤呼吸强度的因素之一。有研究表明,排水引起的地下水位下降,致使土壤有机质暴露于更多的有氧环境中,进而加速有机质氧化分解,导致泥炭层收缩,而泥炭厚度的降低则会引起土壤有机碳储量的降低[38]。此外,刈割所导致的凋落物输入减少,可能会引起可降解基质减少[39],同时也会降低土壤微生物生物量[40],进而降低土壤异养呼吸。可见,水位下降与刈割主要通过影响微生物生长所需的底物可利用性进而影响土壤异养呼吸。有研究表明,微生物活性随土壤含水量的降低而降低,进而影响土壤呼吸,而随着时间推移微生物活性降低逐渐减缓,趋于逐渐适应干旱胁迫[41]。本研究采样时距样地设置仅为1年,而持续水位下降条件下土壤呼吸的变化情况还有待进一步研究。
水位下降与刈割二者交互作用对土壤硝态氮含量有显著影响,与其他的研究结果一致[42-43]。有研究指出,水位下降可以促进土壤氮素矿化,从而促进硝化与反硝化过程,增加N2O的释放量;且高寒湿地水位在-2 cm至-20 cm之间有利于硝化和反硝化的共同进行,产生较多的N2O,进而显著降低土壤硝态氮含量[43]。当水位进一步下降时,则不利于反硝化细菌活动,硝态氮含量应有所增加。然而,本研究中硝态氮含量持续降低,究其原因,可能是在有氧的条件下,发生了氨氧化微生物的反硝化作用,将亚硝酸盐还原为N2O,进而显著降低土壤中硝态氮含量[44]。
3.2 模拟水位下降与刈割对AOA-amoA、AOB-amoA、nirK和nirS基因丰度的影响模拟水位下降显著降低土壤含水量,水的可利用性会显著影响土壤养分循环[45]。有人在对若尔盖湿地进行研究时发现,土壤水分含量与土壤有机碳、氮含量正相关[46],而土壤碳、氮含量被认为是土壤微生物生长的主要限制因素[47]。本研究中模拟水位下降与刈割均对土壤氨氧化微生物基因丰度无显著影响,但刈割、刈割与水位下降二者交互作用对AOA-amoA与AOB-amoA基因丰度比有显著影响。AOA-amoA与AOB-amoA基因丰度比与铵态氮含量负相关,且AOB-amoA基因的相对丰度对刈割显著响应,说明AOB可能在湿地土壤硝化过程中占主导作用。AOB-amoA基因的相对丰度在深水位刈割处理中最高,主要与水位下降所引起的土壤含水量显著降低有关,AOB是好氧微生物,水分通过控制土壤中O2的扩散而间接影响好氧微生物的活性[48];同时,刈割降低了地上部分覆盖面积,提高土壤通气性,减小O2的扩散阻力,进而促进好氧微生物的生长。
通常情况下,AOB-amoA基因相对丰度的增加会提高反硝化作用的底物硝酸盐含量,进而促进由反硝化微生物驱动的反硝化作用,促进硝酸盐被还原为N2O或N2,导致氮素流失。然而,本研究中反硝化功能基因nirK和nirS丰度无显著变化,但水位下降与刈割二者交互作用显著降低了土壤硝态氮含量,说明有其他途径导致了土壤硝酸盐含量的降低。有研究表明,氨氧化微生物在有氧条件下,会将亚硝酸盐还原为一氧化氮(NO),即发生氨氧化微生物的反硝化作用,且AOB是在有氧的状态下发生氨氧化微生物反硝化作用的主要驱动者[42]。本研究中AOB-amoA基因相对丰度的增加印证了这一观点。
本研究中nirS基因丰度显著高于nirK,与其他研究结果相一致[49]。nirS基因丰度与土壤硝态氮含量显著负相关,说明nirS对水位下降及刈割的响应更敏感,在反硝化过程中可能起着比nirK更为重要的作用。这主要与土壤含水量的显著变化有关。有研究表明,以nirS基因为代表的反硝化微生物群落组成对水分变化响应显著[50]。可见,水分条件是影响土壤反硝化过程功能微生物基因丰度的关键因子。
4 结论(1)土壤水位下降显著降低土壤含水量,限制了可溶性有机碳的有效性,从而间接影响微生物生长所需的底物和能量来源,降低土壤异养呼吸。
(2)水位下降和刈割对氨氧化微生物与反硝化细菌基因丰度无显著影响,但AOB-amoA基因相对丰度对刈割显著响应,表明AOB可能在湿地土壤硝化过程中占主导作用。
(3)随着土壤水位的下降,刈割促进了由AOB主导的氨氧化过程,而反硝化微生物丰度的增加削减了氨氧化产物硝酸盐的积累,继而降低了土壤硝酸盐的含量。
| [1] |
Armas C, Kim J H, Bleby T M, et al. The effect of hydraulic lift on organic matter decomposition, soil nitrogen cycling, and nitrogen acquisition by a grass species[J]. Oecologia, 2012, 168(1): 11-22. DOI:10.1007/s00442-011-2065-2 |
| [2] |
Shuang X, Guo R Q, Ning W, et al. Current status and future prospects of Zoige marsh in eastern Qinghai-Tibet Plateau[J]. Ecological Engineering, 2009, 35(4): 553-562. DOI:10.1016/j.ecoleng.2008.02.016 |
| [3] |
Molina V, Ulloa O, Farías L, et al. Ammonia-oxidizing beta-proteobacteria from the oxygen minimum zone off northern Chile[J]. Applied & Environmental Microbiology, 2007, 73(11): 3547. |
| [4] |
贺纪正, 张丽梅. 氨氧化微生物生态学与氮循环研究进展[J]. 生态学报, 2009, 29(1): 406-415. HE Ji-zheng, ZHANG Li-mei. Advances in ammonia-oxidizing microorganisms and global nitrogen cycle[J]. Acta Ecologica Sinica, 2009, 29(1): 406-415. |
| [5] |
Kowalchuk G A, Stephen J R. Ammonia-oxidizing bacteria:A model for molecular microbial ecology[J]. Annual Review of Microbiology, 2001, 55(1): 485. DOI:10.1146/annurev.micro.55.1.485 |
| [6] |
Leininger S, Urich T, Schloter M, et al. Archaea predominate among ammonia-oxidizing prokaryotes in soils[J]. Nature, 2006, 442(7104): 806-809. DOI:10.1038/nature04983 |
| [7] |
Morales S E, Cosart T, Holben W E. Bacterial gene abundances as indicators of greenhouse gas emission in soils[J]. The ISME Journal, 2010, 4(6): 799. DOI:10.1038/ismej.2010.8 |
| [8] |
Szukics U, Hackl E, Zechmeister-Boltenstern S, et al. Contrasting response of two forest soils to nitrogen input:Rapidly altered NO and N2O emissions and nirK abundance[J]. Biology & Fertility of Soils, 2009, 45(8): 855-863. |
| [9] |
Bao Q, Ju X, Gao B, et al. Response of nitrous oxide and corresponding bacteria to managements in an agricultural soil[J]. Soil Science Society of America Journal, 2012, 76(1): 130. DOI:10.2136/sssaj2011.0152 |
| [10] |
Xiang S, Guo R, Wu N, et al. Current status and future prospects of Zoige marsh in eastern Qinghai-Tibet Plateau[J]. Ecological Engineering, 2009, 35(4): 553-562. DOI:10.1016/j.ecoleng.2008.02.016 |
| [11] |
Wu X, Cao R, Wei X, et al. Soil drainage facilitates earthworm invasion and subsequent carbon loss from peatland soil[J]. Journal of Applied Ecology, 2017. DOI:10.1111/1365-2664.12894 |
| [12] |
Danevcic T, Mandic-Mulec I, Stres B, et al. Emissions of CO2, CH4 and N2O from southern European peatlands[J]. Soil Biology & Biochemistry, 2010, 42(9): 1437-1446. |
| [13] |
李丽, 高俊琴, 雷光春, 等. 若尔盖不同地下水位泥炭湿地土壤有机碳和全氮分布规律[J]. 生态学杂志, 2011, 30(11): 2449-2455. ZHANG Li, GAO Jun-qin, LEI Guang-chun, et al. Distribution patterns of soil organic carbon and total nitrogen in Zoige peatland with different ground water table[J]. Chinese Journal of Ecology, 2011, 30(11): 2449-2455. |
| [14] |
赵宁, 张洪轩, 王若梦, 等. 放牧对若尔盖高寒草甸土壤氮矿化及其温度敏感性的影响[J]. 生态学报, 2014, 34(15): 4234-4241. ZHAO Ning, ZHANG Hong-xuan, WANG Ruo-meng, et al. Effect of grazing intensity on temperature sensitivity of soil nitrogen mineralization in Zoige alpine meadow[J]. Acta Ecologica Sinica, 2014, 34(15): 4234-4241. |
| [15] |
Tian J, Shu C, Chen H, et al. Response of archaeal communities to water regimes under simulated warming and drought conditions in Tibetan Plateau wetlands[J]. Journal of Soils and Sediments, 2015, 15(1): 179-188. DOI:10.1007/s11368-014-0978-1 |
| [16] |
Wu L, Su F, Nie Y, et al. Soil cellulase activity and fungal community responses to wetland degradation in the Zoige Plateau, China[J]. Journal of Mountain Science, 2015, 12(2): 471-482. DOI:10.1007/s11629-014-3183-1 |
| [17] |
Zhang G, Tian J, Jiang N, et al. Methanogen community in Zoige wetland of Tibetan plateau and phenotypic characterization of a dominant uncultured methanogen cluster ZC-I[J]. Environmental Microbiology, 2008, 10(7): 1850-1860. DOI:10.1111/j.1462-2920.2008.01606.x |
| [18] |
Kang H, Jang I, Kim S. Key processes in CH4 dynamics in wetlands and possible shifts with climate change[M]. Netherlands: Springer, 2012, 99-114.
|
| [19] |
郑有坤, 王宪斌, 辜运富, 等. 若尔盖高原湿地土壤氨氧化古菌的多样性[J]. 微生物学报, 2014, 54(9): 1090-1096. ZHENG You-kun, WANG Xian-bin, GU Yun-fu, et al. Diversity of ammonia-oxidizing archaea in Tibetan Zoige plateau wetland[J]. Acta Microbiologica Sinica, 2014, 54(9): 1090-1096. |
| [20] |
Wu L, Nie Y, Yang Z, et al. Responses of soil inhabiting nitrogen-cycling microbial communities to wetland degradation on the Zoige Plateau, China[J]. Journal of Mountain Science, 2016, 13(12): 2192-2204. DOI:10.1007/s11629-016-4004-5 |
| [21] |
蔡倩倩, 郭志华, 胡启鹏, 等. 若尔盖高寒嵩草草甸湿地不同水分条件下土壤有机碳的垂直分布[J]. 林业科学, 2013, 49(3): 9-16. CAI Qian-qian, GUO Zhi-hua, HU Qi-peng, et al. Vertical distribution of soil organic carbon and carbon storage under different hydrologic conditions in Zoigê alpine Kobresia meadows wetland[J]. Scientia Silvae Sinicae, 2013, 49(3): 9-16. DOI:10.11707/j.1001-7488.20130302 |
| [22] |
Chen H, Wu N, Yao S P, et al. Diurnal variation of methane emissions from an alpine wetland on the eastern edge of Qinghai-Tibetan Plateau[J]. Environmental Monitoring and Assessment, 2010, 164(1/2/3/4): 21-28. |
| [23] |
Tian J Q, Zhu Y B, Kang X M, et al. Effects of drought on the archaeal community in soil of the Zoige wetlands of the Qinghai-Tibetan plateau[J]. European Journal of Soil Biology, 2012, 52(5): 84-90. |
| [24] |
Yang G, Chen H, Wu N, et al. Effects of soil warming, rainfall reduction and water table level on CH4 emissions from the Zoige peatland in China[J]. Soil Biology & Biochemistry, 2014, 78: 83-89. |
| [25] |
Zhao J, He K, Peng Y, et al. Net neutral effects of a generalist vertebrate predator on seed production result from simultaneous suppression of plant antagonists and mutualists[J]. Basic & Applied Ecology, 2016, 17(4): 344-351. |
| [26] |
Kurola J, Salkinoja-Salonen M, Aarnio T, et al. Activity, diversity and population size of ammonia-oxidizing bacteria in oil-contaminated land farming soil[J]. FEMS Microbiology Letters, 2005, 250(1): 33-38. DOI:10.1016/j.femsle.2005.06.057 |
| [27] |
Brown J R, Blankinship J C, Niboyet A, et al. Effects of multiple global change treatments on soil N2O fluxes[J]. Biogeochemistry, 2012, 109(1): 85-100. |
| [28] |
Touratier F, Legendre L, Vezina A. Model of copepod growth influenced by the food carbon:Nitrogen ratio and concentration, under the hypothesis of strict homeostasis[J]. Journal of Plankton Research, 1999, 21(6): 1111-1132. DOI:10.1093/plankt/21.6.1111 |
| [29] |
Rotthauwe J H, Witzel K P, Liesack W. The ammonia monooxygenase structural gene amoA as a functional marker:Molecular fine-scale analysis of natural ammonia-oxidizing populations[J]. Applied & Environmental Microbiology, 1997, 63(12): 4704-4712. |
| [30] |
Braker G, Ayala-del-Rio H L, Devol A H, et al. Community structure of denitrifiers, bacteria, and archaea along redox gradients in pacific northwest marine sediments by terminal restriction fragment length polymorphism analysis of amplified nitrite reductase(nirS) and 16S rRNA genes[J]. Applied & Environmental Microbiology, 2001, 67(4): 1893-1901. |
| [31] |
Avrahami S, Conrad R, Braker G. Effect of soil ammonium concentration on N2O release and on the community structure of ammonia oxidizers and denitrifiers[J]. Applied & Environmental Microbiology, 2002, 68(11): 5685-5692. |
| [32] |
Luo Y Q, Hui D F, Zhang D Q. Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems:A meta-analysis[J]. Ecology, 2006, 87(1): 53-63. DOI:10.1890/04-1724 |
| [33] |
Trumbore S. Carbon respired by terrestrial ecosystems-recent progress and challenges[J]. Global Change Biology, 2006, 12(2): 141-153. DOI:10.1111/gcb.2006.12.issue-2 |
| [34] |
朱先进, 于贵瑞, 王秋凤, 等. 典型森林和草地生态系统呼吸各组分间的相互关系[J]. 生态学报, 2013, 33(21): 6925-6934. ZHU Xian-jin, YU Gui-rui, WANG Qiu-feng, et al. The interaction between components of ecosystem respiration in typical forest and grassland ecosystems[J]. Acta Ecologica Sinica, 2013, 33(21): 6925-6934. |
| [35] |
Moyano F E, Manzoni S, Chenu C. Responses of soil heterotrophic respiration to moisture availability:An exploration of processes and models[J]. Soil Biology & Biochemistry, 2013, 59: 72-85. |
| [36] |
Casals P, Romanyà J, Cortina J, et al. CO2 efflux from a Mediterranean semi-arid forest soil:I. seasonality and effects of stoniness[J]. Biogeochemistry, 2000, 48(3): 283-306. DOI:10.1023/A:1006208305082 |
| [37] |
陈全胜, 李凌浩, 韩兴国, 等. 水分对土壤呼吸的影响及机理[J]. 生态学报, 2003, 23(5): 972-978. CHEN Quan-sheng, LI Ling-hao, HAN Xing-guo, et al. Effects of water content on soil respiration and the mechanisms[J]. Acta Ecological Sinica, 2003, 23(5): 972-978. |
| [38] |
周文昌. 人类活动对若尔盖高原泥炭地碳通量和碳储量的影响[D]. 北京: 中国林业科学研究院, 2015. ZHOU Wen-chang. Effects of human activities on carbon fluxes and storage in the Zoige peatland of the Qinghai-Tibet Plateau[D]. Beijing:Chinese Academy of Forestry, 2015. http://cdmd.cnki.com.cn/Article/CDMD-82201-1015622495.htm |
| [39] |
Arnold K V, Nilsson M, Hanell B, et al. Fluxes of CO2, CH4 and N2O from drained organic soils in deciduous forests[J]. Soil Biology & Biochemistry, 2005, 37(6): 1059-1071. |
| [40] |
Li Y Q, Xu M, Sun O J, et al. Effects of root and litter exclusion on soil CO2 efflux and microbial biomass in wet tropical forests[J]. Soil Biology & Biochemistry, 2004, 36(12): 2111-2114. |
| [41] |
富宏霖, 王生荣, 韩士杰, 等. 土壤干湿交替对长白山阔叶红松林土壤微生物活性与区系的影响[J]. 东北林业大学学报, 2009, 37(7): 80-81. FU Hong-lin, WANG Sheng-rong, HAN Shi-jie, et al. Influence of wet/dry cycle on soil microbial activity and community flora in broadleaved Korean pine forest in Changbai Mountains[J]. Journal of Northeast Forestry University, 2009, 37(7): 80-81. |
| [42] |
Goldberg S D, Knorr K H, Blodau C, et al. Impact of altering the water table height of an acidic fen on N2O and NO fluxes and soil concentrations[J]. Global Change Biology, 2010, 16(1): 220-233. DOI:10.1111/(ISSN)1365-2486 |
| [43] |
王冬雪, 高永恒, 安小娟, 等. 青藏高原高寒湿地温室气体释放对水位变化的响应[J]. 草业学报, 2016, 25(8): 27-35. WANG Dong-xue, GAO Yong-heng, AN Xiao-juan, et al. Responses of greenhouse gas emissions to water table fluctuations in an alpine wetland on the Qinghai-Tibetan Plateau[J]. Acta Prataculturae Sinica, 2016, 25(8): 27-35. DOI:10.11686/cyxb2016012 |
| [44] |
Wang Q, Zhang L M, Shen J P, et al. Nitrogen fertilizer-induced changes in N2O emissions are attributed more to ammonia-oxidising bacteria rather than archaea as revealed using 1-octyne and acetylene inhibitors in two arable soils[J]. Biology and Fertility of Soils, 2016, 52(8): 1163-1171. DOI:10.1007/s00374-016-1151-3 |
| [45] |
Singh J S, Kashyap A. Dynamics of viable nitrifier community, N-mineralization and nitrification in seasonally dry tropical forests and savanna[J]. Microbiological Research, 2006, 161(2): 169-179. DOI:10.1016/j.micres.2005.07.009 |
| [46] |
Tian Y, Xiong M, Song G. Restoration succession of wetland soils and their changes of water and nutrient in Ruoergai Plateau[J]. Chinese Journal of Ecology, 2005, 24(1): 21-25. |
| [47] |
Kraigher B, Stres B, Hacin J, et al. Microbial activity and community structure in two drained fen soils in the Ljubljana Marsh[J]. Soil Biology & Biochemistry, 2006, 38(9): 2762-2771. |
| [48] |
章燕平. 环境因素对菜地土壤氮素转化及其生物学特性的影响[D]. 杭州: 浙江大学, 2010. ZHANG Yan-ping. Effects of environmental factors on nitrogen transformation and soil biological characteristics in protected vegetable soils[D]. Hangzhou:Zhejiang University, 2010. http://d.wanfangdata.com.cn/Thesis/Y1646559 |
| [49] |
Ligi T, Truu M, Truu J, et al. Effects of soil chemical characteristics and water regime on denitrification genes(nirS, nirK, and nosZ) abundances in a created riverine wetland complex[J]. Ecological Engineering, 2014, 72: 47-55. DOI:10.1016/j.ecoleng.2013.07.015 |
| [50] |
刘若萱, 贺纪正, 张丽梅. 稻田土壤不同水分条件下硝化/反硝化作用及其功能微生物的变化特征[J]. 环境科学, 2014, 1(11): 4275-4283. LIU Ruo-xuan, HE Ji-zheng, ZHANG Li-mei. Response of nitrification/denitrification and their associated microbes to soil moisture change in paddy soil[J]. Environmental Science, 2014, 1(11): 4275-4283. |
 2017, Vol. 36
2017, Vol. 36