文章信息
- 赵颖, 张金波, 蔡祖聪
- ZHAO Ying, ZHANG Jin-bo, CAI Zu-cong
- 添加硝化抑制剂、秸秆及生物炭对亚热带农田土壤N2O排放的影响
- Effects of nitrification inhibitor, crop residues, and biochar applications on N2O emissions by subtropical agricultural soils
- 农业环境科学学报, 2018, 37(5): 1023-1034
- Journal of Agro-Environment Science, 2018, 37(5): 1023-1034
- http://dx.doi.org/10.11654/jaes.2017-1265
文章历史
- 收稿日期: 2017-09-15
- 录用日期: 2017-12-19
2. 虚拟地理环境教育部重点实验室(南京师范大学), 南京 210023;
3. 江苏省地理环境演化国家重点实验室培育建设点, 南京 210023;
4. 江苏省地理信息资源开发与利用协同创新中心, 南京 210023
2. Key Laboratory of Virtual Geographic Environment(Nanjing Normal University), Ministry of Education, Nanjing 210023, China;
3. State Key Laboratory Cultivation Base of Geographical Environment Evolution(Jiangsu Province), Nanjing 210023, China;
4. Jiangsu Center for Collaborative Innovation in Geographical Information Resource Development and Application, Nanjing 210023, China
氧化亚氮(N2O)是一种重要的温室气体,百年尺度上的全球增温势是CO2的298倍[1],也可对平流层臭氧产生严重的破坏作用[2]。从工业革命前至2012年,大气中N2O浓度增加了约20%,达到了325 μL·m-3 [3]。因而,N2O的排放及其环境影响备受各界关注。土壤、沉积物和水体是N2O的主要自然排放源。土壤产生的N2O占自然来源的60%以及总产生量的37%左右[4],而农业生产则贡献了全球人为排放N2O的60%[1, 5]。人为活动引起的N2O排放增加主要由氮的输入增加引起。肥料的施用量、施肥方式以及农业调控措施对农田土壤N2O的排放具有重要的影响[6-7]。常用的农田土壤调控管理措施对N2O的排放具有不同程度的影响。硝化抑制剂可直接减少硝化菌产生N2O,或间接降低反硝化底物NO3-的可利用性,从而减少N2O的排放[8]。施用矿质氮肥的同时添加硝化抑制剂是一种有效的延缓农田土壤硝化作用、减少N2O排放的方法[9]。秸秆还田作为一种农田土壤调控措施,能够维持土壤有机质,减少土壤侵蚀[10]。然而,根据土壤的通气状况以及无机氮和易分解有机碳的供应,添加秸秆可能会因改变硝化和反硝化过程而增加N2O的排放,从而导致土壤N损失的增加[11-13],因此,秸秆还田固碳产生的气候效应有可能因N2O排放的增加而被抵消。此外,农田土壤添加生物炭也成为近年出现的新型管理方式。生物炭中的碳非常稳定,能够长期固存于土壤中[14],并且具有提高土壤肥力、改善土壤理化性质的潜力[15-16]。生物炭对土壤N2O产生的影响的研究结果并不一致[17-24]。一方面,如果生物炭的添加导致N2O和CO2产生的增加,那么生物炭对土壤长期碳固存的正效益可能会受到一定程度的抵消;另一方面,如果生物炭并未增加N2O和CO2的产生,那么可以在增加碳储存的同时降低温室气体排放而形成双倍效益。然而,土壤调控措施对N2O排放的影响效果在不同土壤中存在差异。尽管目前的文献对硝化抑制剂减少N2O排放的作用的报道较一致,但在不同土壤类型中的抑制效率差异较大[25-27]。很多研究表明,对不同类型的土壤进行秸秆还田能够增加农田土壤N2O的排放,但同时也有研究得出秸秆还田能够降低土壤N2O排放或没有影响的结论[10, 28]。生物炭也同样如此,对N2O并不总是表现为减排效应,也可导致一些土壤N2O排放的增加[18-19, 21-22]。土壤类型和性质不同,其自身的氮转化过程特点也不同,这可能是影响土壤氮素调控措施作用效果的重要因素,但是目前尚缺少直接的研究证据。
我国幅员辽阔,自然条件复杂,农业历史悠久,土壤类型繁多。土壤类型的形成、分布与其所处的综合自然环境密切相关。一般认为,发育良好的地带性土壤与其所处的自然环境相适应,通常具有较强的土壤氮素保持能力。如亚热带湿润地区,发育良好的土壤(如红壤)呈酸性,硝化速率通常远低于矿化速率,无机氮以铵态氮为主,因而硝态氮淋溶和径流损失的风险较小,并且较低的pH环境下氨挥发也较低,土壤能够有效地保持氮素[29]。相对于亚热带地区发育良好的地带性土壤,由紫色砂岩页岩发育而来的紫色土是亚热带地区的一种非地带性土壤,广泛分布于长江上游丘陵地区,是中国西南地区重要的旱作土壤之一[30]。紫色土硝化作用较强,土壤无机氮以硝态氮为主[31],氮肥利用率低、损失严重,具有潜在的环境风险。红壤和紫色土均为我国亚热带地区重要的农业土壤,其特点各不相同,N2O的排放量也存在明显的差异,并且因氮肥施用而造成的N2O排放量也不尽相同。如果能够根据亚热带地区不同类型土壤的氮转化过程特点,并结合当地气候和作物等实际条件,筛选出适合的农业调控措施,对于最大程度地减少土壤N2O的排放具有重要的意义。目前我国关于农田土壤N2O排放的研究仍主要集中在东北和华北地区,虽已有学者分别研究了红壤和紫色土地区N2O的排放特征,但多数是针对氮肥品种和施肥方式的影响[6, 7, 32-35],鲜有研究对比不同土壤添加物对这两种典型亚热带农田土壤N2O排放的影响。因此,本研究通过实验室培养试验,研究了短期添加硝化抑制剂、生物炭和秸秆三种土壤添加物对红壤和紫色土农田土壤施氮后N2O排放的影响,同时研究了长期添加秸秆,并在秸秆不同分解阶段添加氮肥后N2O的排放情况,以期为根据土壤类型和性质合理选择氮素调控措施提供理论基础。
1 材料与方法 1.1 供试土壤为了能够更好地理解硝化抑制剂、生物炭和秸秆对亚热带地带性和非地带性农田土壤N2O排放的影响,本试验选择了地带性的酸性红壤和非地带性的石灰性紫色土作为供试土壤。紫色土采自中国科学院四川盐亭紫色土农业生态试验站(SC),红壤采自江西省鹰潭市龙虎山(JX)。四川地区是典型亚热带湿润季风气候,年平均降水量1157 mm,年平均气温17.3 ℃。江西地区同属亚热带湿润季风气候,年平均降水量为1785 mm,年平均气温18.4 ℃。供试紫色土发育于紫砂岩,土壤分类为始成土,红壤发育于第三纪红砂岩,土壤分类为淋溶土[36]。土壤样品均采自0~20 cm耕层。所有土壤样品采集后,挑根、过2 mm筛并混匀,以降低土壤异质性,在进行培养试验前密封储存于4 ℃。供试土壤的理化性质见表 1。
本研究设置2个试验,试验1主要研究各处理措施实施后短期(24 h)内对农田土壤N2O排放的影响;试验2主要研究不同秸秆添加较长时间后(3个月和6个月,即1个生长季)对农田土壤N2O排放的影响。
试验1:每个土壤设置6个处理:①对照(CK),②添加苜蓿(NA),③添加水稻秸秆(NS),④添加甘蔗渣(NBa),⑤添加硝化抑制剂(NC),⑥添加水稻秸秆所制成的生物炭(NB)。硝化抑制剂为2-氯-6-三氯甲基吡啶,浙江奥复托化工有限公司生产,淡黄色乳油,含量24%。秸秆和生物炭粉碎后过0.3 mm筛。三种秸秆和生物炭的主要性质见表 2。称取20 g(烘干重计)土壤于一系列250 mL三角瓶中,每个处理设3个重复,其中苜蓿、水稻秸秆、甘蔗渣和生物炭的物料加入量为0.2 g(相当于1%土壤质量),物料与土壤混合均匀,在25 ℃培养箱中预培养12 h。预培养结束后,每个三角瓶中均匀加入2 mL的NH4NO3溶液,添加量为60 mg N·kg-1。NC处理同时添加所施氮量2.5‰的三氯甲基吡啶。然后,用蒸馏水将土壤水分含量调节至田间最大持水量(WHC)的60%,继续在25 ℃下培养24 h。分别于加氮后第0.5、6、12 h和24 h加入2 mol·L-1 KCl溶液(液土比5:1)浸提土壤以测定无机氮浓度,并于第6、12 h和24 h进行气体的采集。
试验2:设置4个处理,即对照(CK)、添加苜蓿(NA)、添加水稻秸秆(NS)和添加甘蔗渣(NBa),物料的添加量与试验1相同,并设置添加物料后预培养3个月和6个月的两组,期间定期补充蒸馏水以保持含水率为60% WHC。预培养时间达到3个月和6个月后,在每个三角瓶中均匀加入2 mL的NH4NO3溶液,添加量为60 mg N·kg-1,在25 ℃下培养24 h。分别于加氮后第0.5、6、12 h和24 h加入2 mol·L-1 KCl溶液(液土比5:1)浸提土壤以测定无机氮浓度,并于第6、12 h和24 h进行气体的采集。
1.3 气体的采集与测定在添加NH4NO3溶液后的第6、12 h和24 h分别进行气体样品的采集。进行气体样品采集前,用硅胶塞密封三角瓶,接至固定装置抽真空并重新充入新鲜空气,反复三次。将置换好空气的三角瓶在25 ℃下密封培养4 h,分别在密封培养开始(0 h)和结束(4 h)时,使用气密性注射器各采集20 mL气体,并测定其中N2O的浓度。每个采样时间点的N2O排放通量即是密封4 h的N2O平均排放速率。
气体中N2O的浓度测定采用装有63Ni电子捕获检测器(ECD)的Agilent 7890A气相色谱仪测定(Agilent,美国)。色谱柱为80/100目的Porapak Q填充柱,柱温和检测器温度分别为65 ℃和300 ℃。载气为95% Ar+5%CH4,流速为40 mL·min-1。
1.4 土壤采样及测定土壤无机氮含量的测定方法参见《土壤农业化学分析方法》[38]。土壤经2 mol·L-1 KCl提取后在25 ℃下以250 r·min-1转速振荡1 h。提取液经滤纸过滤后,分别使用MgO和定氮合金对其进行蒸馏以测定NH4+和NO3-的浓度。先取用一定比例的提取液加入MgO进行蒸馏以测定NH4+浓度,随后向蒸馏管中加入定氮合金继续蒸馏以测定NO3-浓度。NH4+和NO3-的浓度均由H2SO4滴定确定。
1.5 数据计算与统计分析N2O排放通量计算公式如下:
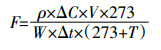
式中:F为N2O排放通量,μg·kg-1·h-1;ρ为标准状况下N2O密度,kg·m-3;ΔC为两次采样间隔(0 h和4 h)的气体浓度差,μL·m-3;V为培养三角瓶的气体有效体积,m3;T为培养温度,℃;Δt为培养时间,h,本研究为4 h;W为烘干土质量,kg。N2O累积排放量为相邻两次测定的N2O排放通量的平均值与时间间隔乘积的累积值,μg·kg-1。
所有统计分析均使用SPSS 19.0(IBM Inc.,美国)软件进行。不同措施对土壤N2O排放的影响差异采用ANOVA和LSD法进行检验。
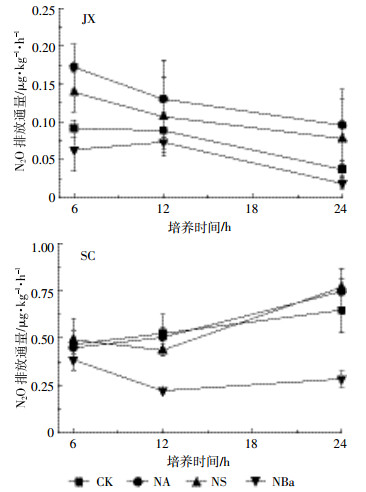
2 结果与分析 2.1 短期内不同调控措施对N2O排放通量和累积排放量的影响从图 1可以看出,JX土壤的N2O排放通量低于SC土壤。两种土壤CK、NC和NB处理均在6 h时出现N2O排放峰值。NC处理两种土壤的N2O排放通量均低于CK。NB处理两种土壤N2O排放通量均高于CK。在JX土壤中添加三种不同秸秆后,只有NS处理的N2O排放通量高于CK,并且在6 h时排放通量出现峰值,而NA和NBa均低于CK且随时间变化不明显。对于SC土壤,三种秸秆处理后的N2O排放通量均高于CK处理,而NA处理中N2O排放通量随时间延长而上升,NS处理的N2O排放通量在6 h出现峰值,并随培养时间延长而下降,NBa处理下的N2O排放通量则变化不明显。

|
| 图 1 短期施加不同土壤添加物后土壤N2O排放通量的变化 Figure 1 N2O fluxes during the incubation of the studied soils with different soil additives application |
加氮培养期间,JX土壤CK、NA、NS、NBa、NC和NB 6个处理的N2O累积排放量分别为7.54、1.82、18.95、1.17、4.49 μg·kg-1和10.70 μg·kg-1;而SC土壤6个处理中的累积排放量分别为24.00、72.15、69.30、31.12、9.21 μg·kg-1和30.82 μg·kg-1(图 2)。SC土壤各处理的N2O排放量均高于JX土壤。NC处理后两种土壤的N2O累积排放量均显著低于CK(P<0.05),分别降低了40%(JX)和62%(SC);而NB处理下,JX土壤的N2O累积排放量显著升高(P<0.05),SC土壤中则虽有升高,但差异不显著(P>0.05)。添加秸秆处理后,JX土壤NA和NBa处理下的N2O累积排放量均显著低于CK(P<0.05),而NS处理下则显著升高(P<0.05)。SC土壤NA和NS处理后N2O累积排放量显著高于CK(P<0.01),NBa处理下N2O累积排放量较CK有所升高,但差异不显著(P>0.05,图 2)。
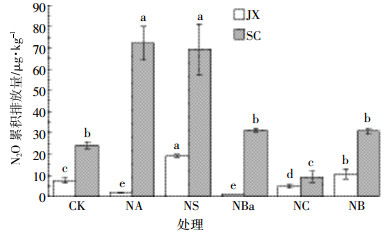
|
| 误差线表示标准差,不同字母表示每种土壤各处理间的显著性差异(P < 0.05)。下同 Error bars represent standard deviations. Different lowercase letters indicate significant difference between treatments in each soil (P < 0.05). The same below 图 2 短期施加不同土壤添加物后土壤N2O的累积排放量 Figure 2 N2O cumulative emissions from soils with different soil additives application |
两种土壤添加秸秆并经过3个月和6个月分解,加氮后各处理中N2O的排放通量均低于添加初期(图 3和图 4)。经3个月预培养后,加氮培养期间,JX土壤的N2O排放通量为NA>NS>CK>NBa,CK和NBa的N2O排放通量在12 h时达到峰值,而NA和NS的峰值则出现在6 h(图 3)。然而在SC土壤中,CK、NA和NS处理下N2O排放通量随时间延长而升高,NBa处理则在6 h出现峰值(图 3)。在预培养6个月后,JX土壤CK、NA和NBa处理N2O排放通量随培养时间延长而降低,而NS处理下N2O排放通量在24 h时最大(图 4)。SC土壤中,4个处理下N2O排放通量均随培养时间延长而升高(图 4)。
|
| 图 3 添加秸秆3个月时加氮后土壤N2O排放通量的变化 Figure 3 N2O fluxes from soils after addition of nitrogen at 3 months after incorporation of crop residues |

|
| 图 4 添加秸秆6个月时加氮后土壤N2O排放通量的变化 Figure 4 N2O fluxes from soils after addition of nitrogen at 6 months after incorporation of crop residues |
土壤添加秸秆并经过3个月和6个月培养后,N2O累积排放量较添加初期有所下降(图 5和图 6)。添加秸秆并经过3个月预培养后,加氮培养期间JX土壤NA和NS处理中N2O累积排放量分别为2.78 μg·kg-1和2.27 μg·kg-1,均显著高于CK(1.57 μg·kg-1,P<0.05),而NBa处理下N2O累积排放量(1.15 μg·kg-1)与CK并无显著差异(P>0.05,图 5)。6个月预培养后,NA和NBa处理则显著增加了JX土壤N2O的累积排放量(P<0.05),分别为2.85 μg·kg-1和2.82 μg·kg-1,但NS处理(2.17 μg·kg-1)与CK(2.33 μg·kg-1)处理间差异不显著(P>0.05,图 6)。SC土壤在经过3个月预培养后,NBa处理显著降低了N2O累积排放量(P<0.05),为6.01 μg·kg-1,而NA和NS处理下的累积排放量分别为11.69 μg·kg-1和11.47 μg·kg-1,与CK(11.39 μg·kg-1)处理间无显著差异(P>0.05,图 5);但6个月后,SC土壤CK处理的N2O累积排放量为9.55 μg·kg-1,NA和NS处理显著增加了N2O的累积排放量(P<0.05),分别为10.88 μg·kg-1和10.36 μg·kg-1,而NBa仍然显著降低了SC土壤的N2O累积排放量(P<0.05),仅为6.85 μg·kg-1(图 6)。
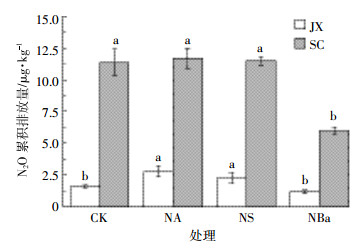
|
| 图 5 添加秸秆3个月时加氮后土壤N2O的累积排放量 Figure 5 N2O cumulative emissions from soils after addition of nitrogen at 3 months after incorporation of crop residues |
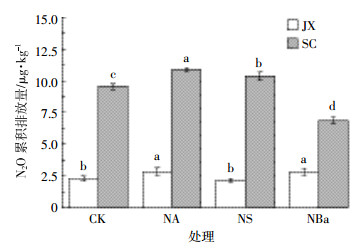
|
| 图 6 添加秸秆6个月时加氮后土壤N2O的累积排放量 Figure 6 N2O cumulative emissions from soils after addition of nitrogen at 6 months after incorporation of crop residues |
短期添加硝化抑制剂、秸秆和生物炭,随着添加氮肥后培养时间的延长,两种土壤各处理的NH4+含量下降,而NO3-含量则逐渐上升,表明发生了净硝化作用。然而NS处理例外,在两种土壤中其无机氮含量均显著下降,表明发生了净同化作用(图 7)。SC土壤中无机氮含量的变化程度大于JX土壤。培养结束后,NC处理的SC土壤中,NH4+含量高于CK处理,而NO3-含量则显著低于CK处理;但硝化抑制剂对JX土壤的影响并不显著。NB处理的土壤在培养结束时,JX土壤中NH4+含量略高于CK处理,而在SC土壤中则低于CK;JX土壤的NO3-含量与CK相比无明显变化,但在SC土壤中则高于CK。NBa处理培养结束时两种土壤NH4+含量低于CK处理,而NO3-含量与CK相比变化不明显。NS处理显著降低了两种土壤的无机氮含量。NA处理后,土壤无机氮浓度的变化趋势与NBa类似。
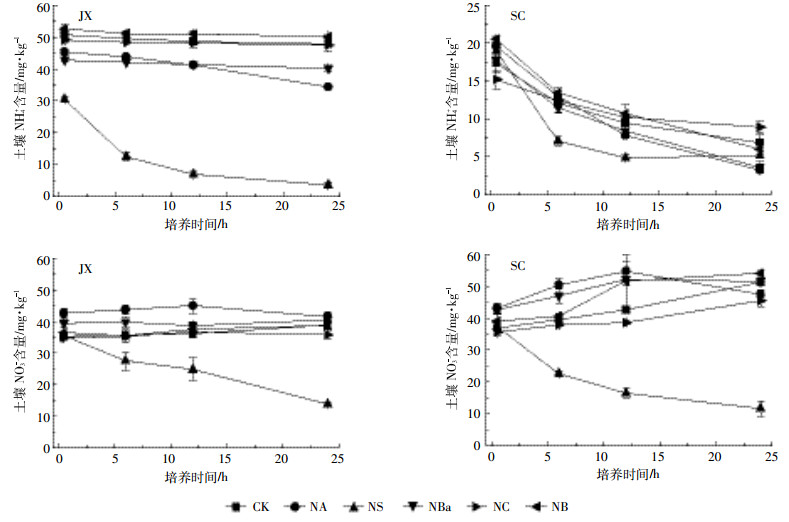
|
| 图 7 短期施加不同添加物并添加NH4NO3后土壤无机氮含量的变化 Figure 7 Changes of soil inorganic N contents after short-term application of different soil additives with addition of NH4NO3 |
在施加秸秆3个月和6个月时添加NH4NO3,其土壤NH4+和NO3-含量随加氮后培养时间的变化趋势与短期添加类似,并且在JX土壤中的变化不如SC土壤显著(图 8)。长期添加秸秆,土壤无机氮含量随秸秆分解时间延长而增加,即6个月时各处理的无机氮含量高于3个月时,尤其是NO3-含量。JX土壤添加秸秆3个月和6个月时加氮,其土壤NO3-含量均为CK>NA>NS≈NBa;而SC土壤在添加秸秆6个月后加氮,其NO3-含量为NA>CK>NS>NBa(图 8)。从图 7和图 8可以看出,短期添加时,甘蔗渣对无机氮含量的影响效果最弱,而水稻秸秆最强,苜蓿的影响效果则在二者之间;秸秆的影响在短期添加时最为显著,随着长期的分解,其影响效果逐渐下降。

|
| 图 8 施加秸秆3个月(3M)和6个月(6M)时添加NH4NO3后土壤无机氮含量的变化 Figure 8 Changes of soil inorganic N contents after addition of NH4NO3 at 3 months(3M)and 6 months(6M)after application of crop residues |
本试验结果表明,在添加的初期阶段(24 h),三氯甲基吡啶可显著降低土壤N2O的排放,尤其是SC土壤。SC土壤在添加NH4NO3溶液后,N2O累积排放量可达24.00 μg·kg-1(CK),而三氯甲基吡啶的添加可降低其累积排放量至9.21 μg·kg-1,抑制程度可达62%,与前人的研究结果[8, 39]相一致。氮肥施加于土壤,2~3周内大部分氮肥即可通过硝化作用转化为易淋失的NO3-[40],进而导致较低的氮保留性。硝化抑制剂可通过抑制土壤氨氧化菌(特别是氨氧化细菌)的活性来延迟NH4+氧化为NO3-[41-42]。因此,硝化抑制剂可通过抑制硝化过程而直接减少硝化作用产生的N2O,或降低土壤NO3-浓度,减少反硝化可利用的底物,从而减少N2O的排放[26]。有研究表明,在60% WHC和25 ℃条件下,反硝化作用可忽略不计[43],因此在本研究中,硝化过程是土壤产生N2O的主要途径[8]。添加三氯甲基吡啶后,SC土壤NO3-浓度降低,三氯甲基吡啶可抑制SC土壤的净硝化作用(图 7)。此外,我们先前的研究表明,SC土壤的硝化速率大于JX土壤,并且以自养硝化为主,而三氯甲基吡啶可显著降低SC土壤的初级自养硝化速率[44]。因此,三氯甲基吡啶对SC土壤N2O排放的影响主要是由于其对自养硝化的抑制效果。添加了三氯甲基吡啶后,JX土壤的NO3-浓度并无显著改变,表明三氯甲基吡啶对JX土壤的净硝化作用无显著影响,N2O累积排放量由7.54 μg·kg-1降低为4.49 μg·kg-1。有研究表明,酸性土壤中异养硝化对N2O排放的贡献远高于自养硝化作用[45-46],然而三氯甲基吡啶并不能有效抑制异养硝化过程,故三氯甲基吡啶对酸性JX土壤N2O的减排效果不如SC土壤明显。因此,对于性质不相同的土壤,硝化抑制剂对N2O排放的影响程度并不一致,若以减少N2O排放为目的,则硝化抑制剂对自养硝化作用较弱的酸性土壤来说并不是最有效的方法。
3.2 添加生物炭对土壤N2O排放的影响添加初期生物炭可显著增加培养期间JX土壤N2O的累积排放量,而SC土壤N2O虽有升高但不显著,这与Clough等[18]、Scheer等[47]以及Yoo等[48]的研究结果相一致。与此相反,也有研究证明生物炭的添加对土壤N2O的排放并无影响或可降低排放[19, 49-51]。我们此前的研究表明,生物炭的添加对两种土壤NO3-产生和消耗过程的初级速率具有一致的影响,生物炭的添加对初级异养硝化速率并无显著影响,但是能够提高初级自养硝化速率[44]。因此,N2O排放的增加可能与土壤自养硝化速率的增加有关。此外,有研究表明,土壤类型、土壤水分条件以及生物炭类型、新鲜度和添加量均可导致生物炭对土壤N2O排放的不同影响[17, 19, 52-53]。Clough等[54]总结认为添加生物炭后N2O排放的升高可归因于生物炭中N的释放对土壤有机质的激发效应,也可能是生物炭的添加增加了土壤含水量并改善了反硝化作用的环境,还可能是由于生物炭为微生物生长提供了无机氮和/或碳源基质。因此,本研究添加生物炭的初始阶段,也可能由于激发效应而导致N2O排放的升高。添加生物炭并配施氮肥培养24 h后,JX土壤的无机氮含量(NH4++NO3-)由只施氮肥的74.05 mg·kg-1增加为88.91 mg·kg-1;而在SC土壤中则由58.12 mg·kg-1增加为60.08 mg·kg-1。因此生物炭的添加为两种土壤中微生物的生长提供了无机氮和碳源,并且在JX土壤中该作用效果更为显著,这与Clough等[54]的研究相一致。长期效果仍需进一步研究,以确定生物炭对两种土壤的长期生态效应。
3.3 添加秸秆对土壤N2O排放的影响从试验结果可看出,添加苜蓿和水稻秸秆短期内(24 h)可显著增加SC土壤中N2O的累积排放量,而甘蔗渣虽然增加了N2O排放但并不显著;JX土壤中水稻秸秆的添加可显著增加N2O累积排放量,但苜蓿和甘蔗渣却显著降低了土壤N2O的排放。因此可以看出,秸秆对土壤N2O排放的影响取决于秸秆类型与性质、土壤质地和理化性质。秸秆的矿化以及随后造成的N2O排放与秸秆的性质,尤其是C/N有关[55]。秸秆在调节土壤N2O的排放中具有多种作用。作为一种有机氮肥,秸秆受到微生物N矿化和硝化作用,从而导致N2O的产生。一般来说,该作用取决于秸秆的氮含量[56-58]。低C/N秸秆具有较高的N含量,往往可导致更大量的N2O排放[55]。秸秆也可作为微生物生长的有机C底物,并因此促进微生物的N同化,该作用通常会导致异养微生物与自养硝化对NH4+的竞争[59-60],进而减少N2O的产生。通常高C/N的秸秆具有此类作用。Chen等[10]分析表明,通常来说,当秸秆C/N<45,秸秆可为土壤微生物群落的生长和增殖提供足够的N源,进而导致净矿化的发生,多余的N可刺激硝化和反硝化作用,从而对土壤N2O排放产生显著的促进作用;当C/N在45~100之间,存在轻微的刺激作用;而当C/N>100,则具有轻微的抑制作用。秸秆C/N较高,秸秆自身的N无法满足秸秆C引起的微生物生长的需求,因此,活性微生物将同化土壤N成为微生物量氮,导致净同化的发生。显然,氮的损耗可降低硝化和反硝化作用,从而减少N2O排放。添加秸秆后土壤异养微生物的生长及其导致的N同化是N2O排放与秸秆C/N呈负相关的基础[57-58]。
然而,从本研究的结果也可看出,C/N的影响也并非绝对的,高C/N的秸秆并不总会降低土壤N2O的排放,这表明秸秆也可能影响土壤无机氮以外的非生物因素[61-62]。例如,添加秸秆后微生物的生长消耗了土壤孔隙中大量的O2,导致土壤微域的好氧环境转变为了厌氧条件,因而反硝化可能取代硝化作用,成为一些土壤孔隙中N2O产生的主要过程,从而增加N2O的排放。异养微生物生长导致的O2消耗与添加的秸秆量呈正相关,即影响程度与输入的秸秆C和N含量呈正相关。此外,秸秆对SC土壤N2O排放的影响效果更为显著,这可能与土壤质地和理化性质有关。根据Chen等[10]的分析,在砂性土中,秸秆还田对土壤N2O排放的影响较小,但在其他土壤类型中,秸秆的添加可显著刺激土壤N2O的排放。从土壤性质可看出,JX土壤属砂性土,因此秸秆对JX土壤N2O排放的影响小于SC土壤。土壤质地对N2O排放的影响可能是通过O2可利用性间接产生的[63-64]。此外,由于添加秸秆对土壤N2O排放的影响在中性和微碱性条件下更加显著[10],也可能使得添加秸秆后SC土壤中N2O的排放更为剧烈。
秸秆对土壤N2O排放的影响也与秸秆添加后的分解时间有关。从本试验结果来看,经过较为长期的分解后,秸秆对土壤N2O排放的影响随着添加时间延长而下降,尤其是C/N相对较低的苜蓿和水稻秸秆,影响程度不如初期时明显,但甘蔗渣在添加6个月后仍能显著降低SC土壤的N2O排放。经过6个月长期培养后,由于秸秆中的植物C和养分被分解,秸秆对微生物活性的刺激效果降低,故对N2O排放的影响程度降低。一般来说,C/N低的秸秆在土壤中更容易矿化分解[65]。此外,秸秆中各类有机化合物,如纤维素、半纤维素、木质素等的含量差异对秸秆的分解转化也有很大的影响[66]。甘蔗渣的组分中含有约40%的纤维素、24%的半纤维素和25%的木质素,其中木质素是最难降解的组分,这导致甘蔗渣不易被矿化[67-68]。因此,从土壤无机氮浓度可看出,较难分解的甘蔗渣,在经过6个月的培养后,对土壤无机氮的同化作用仍较高,氮的损耗可减少N2O的排放。
从上述试验结果综合来看,虽然本研究中添加生物炭后,N2O排放有所上升,但其刺激程度低于秸秆,因此,从降低N2O排放的角度来说,将秸秆制成生物炭也许是一个好的选择。但是,本研究是在室内条件下进行的培养试验,对于野外大田的真实环境还需进一步的研究。此外,本研究并未从微生物学机理的角度来阐释调控措施对N2O排放的影响机制,可进一步从该角度来进行研究。
施肥对土壤N2O的排放具有促进作用[7]。本研究中添加NH4NO3溶液后,短时间内可造成土壤中无机氮的积累,为硝化和反硝化微生物提供充足的底物和能源,进而可增加土壤N2O的排放通量。但是本研究没有设置不加氮对照,不能评估添加氮的激发效应。本研究的重点是以只添加NH4NO3处理为对照,研究添加硝化抑制剂、生物炭和秸秆三种土壤添加物,对红壤和紫色土农田土壤施氮后N2O排放的影响,以期为根据土壤类型和性质合理选择氮素调控措施提供理论基础。
4 结论在对土壤施肥并进行调控时,应当根据土壤类型和性质进行选择。若以减少N2O排放为目的,那么对于碱性土壤选择硝化抑制剂调控是合适的,但对酸性土壤则效果并不显著。生物炭对土壤N2O排放的影响也因土壤性质不同而不同。秸秆的添加在初期分解阶段会显著增加土壤N2O排放,对于环境来说是不利的影响,但随着时间的增加刺激效果会随之下降,因此,客观的评价需要进行长期观察。从本试验来看,若将秸秆转化为生物炭,以稳定的生物炭的C形式添加于土壤,其N2O的排放少于秸秆,对于减少土壤温室气体排放和增加土壤C储存是较为有益的。
| [1] |
IPCC. Climate change 2007: The physical science basis. Contribution of working group Ⅰ to the fourth assessment report of the Intergovernmental Panel on Climate Change[R]. Cambridge: Cambridge University Press, 2007.
|
| [2] |
Ravishankara A R, Daniel J S, Portmann R W. Nitrous oxide(N2O):The dominant ozone-depleting substance emitted in the 21st Century[J]. Science, 2009, 326(5949): 123-125. DOI:10.1126/science.1176985 |
| [3] |
WMO. The state of greenhouse gases in the atmosphere based on global observations through 2012[R]. WMO Greenhouse Gas Bulletin, 2013, 9: 1-4.
|
| [4] |
IPCC. Climate change 2013: The physical science basis. Contribution of working group Ⅰ to the fifth assessment report of the Intergovernmental Panel on Climate Change[R]. Cambridge: Cambridge University Press, 2013.
|
| [5] |
Reay D S, Davidson E A, Smith K A, et al. Global agriculture and nitrous oxide emissions[J]. Nature Climate Change, 2012, 2(6): 410-416. DOI:10.1038/nclimate1458 |
| [6] |
柳文丽, 李锡鹏, 沈茜, 等. 施肥方式对冬小麦季紫色土N2O排放特征的影响[J]. 中国生态农业学报, 2014, 22(9): 1029-1037. LIU Wen-li, LI Xi-peng, SHEN Xi, et al. Effects of fertilizer application regimes on soil N2O emissions in the croplands of purple soil in the Sichuan Basin during wheat season[J]. Chinese Journal of Eco-Agriculture, 2014, 22(9): 1029-1037. |
| [7] |
曾泽彬, 朱波, 朱雪梅, 等. 施肥对夏玉米季紫色土N2O排放及反硝化作用的影响[J]. 土壤学报, 2013, 50(1): 130-136. ZENG Ze-bin, ZHU Bo, ZHU Xue-mei, et al. Effects of fertilization on N2O emission and denitrification in purple soil during summer maize season in the Sichuan Basin[J]. Acta Pedologica Sinica, 2013, 50(1): 130-136. DOI:10.11766/trxb201203010055 |
| [8] |
Lan T, Han Y, Roelcke M, et al. Effects of the nitrification inhibitor dicyandiamide(DCD) on gross N transformation rates and mitigating N2O emission in paddy soils[J]. Soil Biology & Biochemistry, 2013, 67: 174-182. |
| [9] |
Wolt J D. A meta-evaluation of nitrapyrin agronomic and environmental effectiveness with emphasis on corn production in the Midwestern USA[J]. Nutrient Cycling in Agroecosystems, 2004, 69(1): 23-41. DOI:10.1023/B:FRES.0000025287.52565.99 |
| [10] |
Chen H H, Li X C, Hu F, et al. Soil nitrous oxide emissions following crop residue addition:A meta-analysis[J]. Global Change Biology, 2013, 19(10): 2956-2964. DOI:10.1111/gcb.2013.19.issue-10 |
| [11] |
Mitchell R D J, Harrison R, Russell K J, et al. The effect of crop residue incorporation date on soil inorganic nitrogen, nitrate leaching and nitrogen mineralization[J]. Biology and Fertility of Soils, 2000, 32(4): 294-301. DOI:10.1007/s003740000251 |
| [12] |
Miller M N, Zebarth B J, Dandie C E, et al. Crop residue influence on denitrification, N2O emissions and denitrifier community abundance in soil[J]. Soil Biology & Biochemistry, 2008, 40: 2553-2562. |
| [13] |
Begum N, Guppy C, Herridge D, et al. Influence of source and quality of plant residues on emissions of N2O and CO2 from a fertile, acidic Black Vertisol[J]. Biology and Fertility of Soils, 2014, 50(3): 499-506. DOI:10.1007/s00374-013-0865-8 |
| [14] |
Tenenbaum D J. Biochar:Carbon mitigation from the ground up[J]. Environmental Health Perspectives, 2009, 117(2). |
| [15] |
Glaser B, Lehmann J, Zech W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal:A review[J]. Biology and Fertility of Soils, 2002, 35(4): 219-230. DOI:10.1007/s00374-002-0466-4 |
| [16] |
Lehmann J, da Silva J P, Steiner C, et al. Nutrient availability and leaching in an archaeological anthrosol and a ferralsol of the Central Amazon basin:Fertilizer, manure and charcoal amendments[J]. Plant and Soil, 2003, 249(2): 343-357. DOI:10.1023/A:1022833116184 |
| [17] |
Yanai Y, Toyota K, Okazaki M. Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiment[J]. Soil Science and Plant Nutrition, 2007, 53(2): 181-188. DOI:10.1111/j.1747-0765.2007.00123.x |
| [18] |
Clough T J, Bertram J E, Ray J L, et al. Unweathered wood biochar impact on nitrous oxide emissions from a bovine-urine-amended pasture soil[J]. Soil Science Society of America Journal, 2010, 74(3): 852-860. DOI:10.2136/sssaj2009.0185 |
| [19] |
Singh B P, Hatton B J, Singh B, et al. Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils[J]. Journal of Environmental Quality, 2010, 39(4): 1224-1235. DOI:10.2134/jeq2009.0138 |
| [20] |
Castaldi S, Riondino M, Baronti S, et al. Impact of biochar application to a Mediterranean wheat crop on soil microbial activity and greenhouse gas fluxes[J]. Chemosphere, 2011, 85(9): 1464-1471. DOI:10.1016/j.chemosphere.2011.08.031 |
| [21] |
Wang J Y, Zhang M, Xiong Z Q, et al. Effects of biochar addition on N2O and CO2 emissions from two paddy soils[J]. Biology and Fertility of Soils, 2011, 47(8): 887-896. DOI:10.1007/s00374-011-0595-8 |
| [22] |
Cheng Y, Cai Z C, Chang S X, et al. Wheat straw and its biochar have contrasting effects on inorganic N retention and N2O production in a cultivated Black Chernozem[J]. Biology and Fertility of Soils, 2012, 48(8): 941-946. DOI:10.1007/s00374-012-0687-0 |
| [23] |
Wu F P, Jia Z K, Wang S G, et al. Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a Chernozemic soil[J]. Biology and Fertility of Soils, 2013, 49(5): 555-565. DOI:10.1007/s00374-012-0745-7 |
| [24] |
Cayuela M L, Zwieten L V, Singh B P, et al. Biochar's role in mitigating soil nitrous oxide emissions:A review and meta-analysis[J]. Agriculture Ecosystems & Environment, 2014, 191(5): 5-16. |
| [25] |
Barth G, von Tucher S, Schmidhalter U. Influence of soil parameters on the effect of 3, 4-dimethylpyrazole-phosphate as a nitrification inhibitor[J]. Biology and Fertility of Soils, 2001, 34(2): 98-102. DOI:10.1007/s003740100382 |
| [26] |
Akiyama H, Yan X Y, Yagi K, et al. Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils:Meta-analysis[J]. Global Change Biology, 2010, 16(6): 1837-1846. |
| [27] |
Gilsanz C, Báez D, Misselbrook T H, et al. Development of emission factors and efficiency of two nitrification inhibitors, DCD and DMPP[J]. Agriculture, Ecosystems and Environment, 2016, 216: 1-8. DOI:10.1016/j.agee.2015.09.030 |
| [28] |
张冉, 赵鑫, 濮超, 等. 中国农田秸秆还田土壤N2O排放及其影响因素的Meta分析[J]. 农业工程学报, 2015, 31(22): 1-6. ZHANG Ran, ZHAO Xin, PU Chao, et al. Meta-analysis on effects of residue retention on soil N2O emissions and influence factors in China[J]. Transactions of the Chinese Society of Agricultural Engineering, 2015, 31(22): 1-6. DOI:10.11975/j.issn.1002-6819.2015.22.001 |
| [29] |
Zhang J B, Zhu T B, Meng T Z, et al. Agricultural land use affects nitrate production and conservation in humid subtropical soils in China[J]. Soil Biology & Biochemistry, 2013, 62: 107-114. |
| [30] |
Li Q Q, Luo Y L, Wang C Q, et al. Spatiotemporal variations and factors affecting soil nitrogen in the purple hilly area of Southwest China during the 1980s and the 2010s[J]. Science of the Total Environment, 2016, 547(15): 173-181. |
| [31] |
Wang J, Zhu B, Zhang J B, et al. Mechanisms of soil N dynamics following long-term application of organic fertilizers to subtropical rain-fed purple soil in China[J]. Soil Biology & Biochemistry, 2015, 91: 222-231. |
| [32] |
黄晶, 张杨珠, 刘宏斌, 等. 长期不同施肥条件下红壤小麦和玉米季CO2、N2O排放特征[J]. 生态与农村环境学报, 2011, 27(4): 7-13. HUANG Jing, ZHANG Yang-zhu, LIU Hong-bin, et al. CO2 and N2O emissions from red soil during wheat and corn growing seasons under different patterns of long-term fertilization[J]. Journal of Ecology and Rural Environment, 2011, 27(4): 7-13. |
| [33] |
谢义琴, 张建峰, 姜慧敏, 等. 不同施肥措施对稻田土壤温室气体排放的影响[J]. 农业环境科学学报, 2015, 34(3): 578-584. XIE Yi-qin, ZHANG Jian-feng, JIANG Hui-min, et al. Effects of different fertilization practices on greenhouse gas emissions from paddy soil[J]. Journal of Agro-Environment Science, 2015, 34(3): 578-584. DOI:10.11654/jaes.2015.03.022 |
| [34] |
林超文, 刘海涛, 朱波, 等. 控释氮肥对紫色土坡耕地N2O排放量的影响[J]. 西南农业学报, 2016, 29(10): 2427-2431. LIN Chao-wen, LIU Hai-tao, ZHU Bo, et al. Effects of applying controlled release nitrogen on nitrous oxide emission in slope purple soil[J]. Southwest China Journal of Agricultural Sciences, 2016, 29(10): 2427-2431. |
| [35] |
胡磊, 刘韵, 朱波. 不同施肥方式下紫色土N2O与NOx的排放特征[J]. 环境科学, 2017, 38(8): 3442-3450. HU Lei, LIU Yun, ZHU Bo. Characteristics of N2O and NOx emissions from purple soil under different fertilization regimes[J]. Environmental Science, 2017, 38(8): 3442-3450. |
| [36] |
FAO-UNESCO-ISRIC. Soil map of the world. Revised legend. World soil resources report 60[R]. Rome: FAO, 1990.
|
| [37] |
Zhao X, Wang J W, Wang S Q, et al. Successive straw biochar application as a strategy to sequester carbon and improve fertility:A pot experiment with two rice/wheat rotations in paddy soil[J]. Plant and Soil, 2014, 378(1/2): 279-294. |
| [38] |
鲁如坤. 土壤农业化学分析方法[M]. 北京: 中国农业科技出版社, 2000. LU Ru-kun. Soil agro-chemical analyses[M]. Beijing: China Agricultural Science and Technology Press, 2000. |
| [39] |
Chen D L, Suter H C, Islam A, et al. Influence of nitrification inhibitors on nitrification and nitrous oxide(N2O) emission from a clay loam soil fertilized with urea[J]. Soil Biology & Biochemistry, 2010, 42(4): 660-664. |
| [40] |
Huber D M, Warren H L, Nelson D W, et al. Nitrification inhibitors:New tools for food production[J]. BioScience, 1977, 27(8): 523-529. DOI:10.2307/1297812 |
| [41] |
Di H J, Cameron K C, Shen J P, et al. A lysimeter study of nitrate leaching from grazed grassland as affected by a nitrification inhibitor, dicyandiamide, and relationships with ammonia oxidizing bacteria and archaea[J]. Soil Use and Management, 2009, 25(4): 454-461. DOI:10.1111/sum.2009.25.issue-4 |
| [42] |
Guo Y J, Di H J, Cameron K C, et al. Effect of 7-year application of a nitrification inhibitor, dicyandiamide(DCD), on soil microbial biomass, protease and deaminase activities, and the abundance of bacteria and archaea in pasture soils[J]. Journal of Soils and Sediments, 2013, 13(4): 753-759. DOI:10.1007/s11368-012-0646-2 |
| [43] |
Nishio T, Komada M, Arao T, et al. Simultaneous determination of transformation rates of nitrate in soil[J]. Japan Agricultural Research Quarterly, 2001, 35(1): 11-17. DOI:10.6090/jarq.35.11 |
| [44] |
Zhao Y, Wang J, Cai Z C, et al. Short-term effects of nitrapyrin, rice straw and its biochar application on N transformation in soils of humid subtropical China[J]. Acta Agriculturae Scandinavica, Section B:Soil & Plant Science, 2018, 68(5): 448-456. |
| [45] |
Zhang J B, Cai Z C, Zhu T B. N2O production pathways in the subtropical acid forest soils in China[J]. Environmental Research, 2011, 111(5): 643-649. DOI:10.1016/j.envres.2011.04.005 |
| [46] |
Stange C F, Spott O, Arriaga H, et al. Use of the inverse abundance approach to identify the sources of NO and N2O release from Spanish forest soils under oxic and hypoxic conditions[J]. Soil Biology & Biochemistry, 2013, 57: 451-458. |
| [47] |
Scheer C, Grace P R, Rowlings D W, et al. Effect of biochar amendment on the soil-atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia[J]. Plant and Soil, 2011, 345(1): 47-58. |
| [48] |
Yoo G, Kang H J. Effects of biochar addition on greenhouse gas emissions and microbial responses in a short-term laboratory experiment[J]. Journal of Environmental Quality, 2012, 41(4): 1193-1202. DOI:10.2134/jeq2011.0157 |
| [49] |
Bruun E W, Müller-Stöver D, Ambus P, et al. Application of biochar to soil and N2O emissions:Potential effects of blending fast-pyrolysis biochar with anaerobically digested slurry[J]. European Journal of Soil Science, 2011, 62(4): 581-589. DOI:10.1111/ejs.2011.62.issue-4 |
| [50] |
Rogovska N, Laird D, Cruse R, et al. Impact of biochar on manure carbon stabilization and greenhouse gas emissions[J]. Soil Science Society of America Journal, 2011, 75(3): 871-879. DOI:10.2136/sssaj2010.0270 |
| [51] |
Kammann C, Ratering S, Eckhard C, et al. Biochar and hydrochar effects on greenhouse gas(carbon dioxide, nitrous oxide, and methane) fluxes from soils[J]. Journal of Environmental Quality, 2012, 41(4): 1052-1066. DOI:10.2134/jeq2011.0132 |
| [52] |
DeLuca T H, MacKenzie M D, Gundale M J, et al. Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests[J]. Soil Science Society of America Journal, 2006, 70(2): 448-453. DOI:10.2136/sssaj2005.0096 |
| [53] |
Spokas K A, Koskinen W C, Baker J M, et al. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil[J]. Chemosphere, 2009, 77(4): 574-581. DOI:10.1016/j.chemosphere.2009.06.053 |
| [54] |
Clough T J, Condron L M, Kammann C, et al. A review of biochar and soil nitrogen dynamics[J]. Agronomy, 2013, 3(2): 275-293. DOI:10.3390/agronomy3020275 |
| [55] |
Aulakh M S, Doran J W, Walter D T, et al. Crop residue type and placement effects on denitrification and mineralization[J]. Soil Science Society of America Journal, 1991, 55(4): 1020-1025. DOI:10.2136/sssaj1991.03615995005500040022x |
| [56] |
Huang Y, Zou J W, Zheng X H, et al. Nitrous oxide emissions as influenced by amendment of plant residues with different C:N ratios[J]. Soil Biology & Biochemistry, 2004, 36(6): 973-981. |
| [57] |
Garcia-Ruiz R, Baggs E M. N2O emission from soil following combined application of fertiliser-N and ground weed residues[J]. Plant and Soil, 2007, 299(1): 263-274. |
| [58] |
Frimpong K A, Baggs E M. Do combined applications of crop residues and inorganic fertilizer lower emission of N2O from soil?[J]. Soil Use and Management, 2010, 26(4): 412-424. DOI:10.1111/j.1475-2743.2010.00293.x |
| [59] |
Shi W, Norton J M. Effect of long-term, biennial, fall-applied anhydrous ammonia and nitrapyrin on soil nitrification[J]. Soil Science Society of America Journal, 2000, 64(1): 228-234. DOI:10.2136/sssaj2000.641228x |
| [60] |
Burger M, Jackson L E. Microbial immobilization of ammonium and nitrate in relation to ammonification and nitrification rates in organic and conventional cropping systems[J]. Soil Biology & Biochemistry, 2003, 35(1): 29-36. |
| [61] |
Li X, Hu F, Bowman D, et al. Nitrous oxide production in turfgrass systems:Effects of soil properties and grass clipping recycling[J]. Applied Soil Ecology, 2013, 67: 61-69. DOI:10.1016/j.apsoil.2013.03.002 |
| [62] |
Li X, Hu F, Shi W. Plant material addition affects soil nitrous oxide production differently between aerobic and oxygen-limited conditions[J]. Applied Soil Ecology, 2013, 64: 91-98. DOI:10.1016/j.apsoil.2012.10.003 |
| [63] |
Skiba U, Ball B. The effect of soil texture and soil drainage on emissions of nitric oxide and nitrous oxide[J]. Soil Use and Management, 2002, 18(1): 56-60. DOI:10.1079/SUM2002101 |
| [64] |
Singurindy O, Richards B K, Molodovskaya M, et al. Nitrous oxide and ammonia emissions from urine-treated soils:Texture effect[J]. Vadose Zone Journal, 2006, 5(4): 1236-1245. DOI:10.2136/vzj2006.0073 |
| [65] |
Nicolardot B, Recous S, Mary B. Simulation of C and N mineralisation during crop residue decomposition:A simple dynamic model based on the C:N ratio of the residues[J]. Plan and Soil, 2001, 228(1): 83-103. DOI:10.1023/A:1004813801728 |
| [66] |
Gul S, Whalen J. Plant life history and residue chemistry influences emissions of CO2 and N2O from soil:Perspectives for genetically modified cell wall mutants[J]. Critical Reviews in Plant Sciences, 2013, 32(5): 344-368. DOI:10.1080/07352689.2013.781455 |
| [67] |
Saha B C. Hemicellulose bioconversion[J]. Journal of Industrial Microbiology and Biotechnology, 2003, 30(5): 279-291. DOI:10.1007/s10295-003-0049-x |
| [68] |
Dong X Q, Yang J S, Zhu N, et al. Sugarcane bagasse degradation and characterization of three white-rot fungi[J]. Bioresource Technology, 2013, 131: 443-451. DOI:10.1016/j.biortech.2012.12.182 |
 2018, Vol. 37
2018, Vol. 37