2. 辽宁省城市生态重点实验室, 沈阳 110167;
3. 沈阳工程学院辽宁省洁净燃烧发电与供热技术重点实验室, 沈阳 110136;
4. 中国科学院沈阳应用生态研究所, 沈阳 110016;
5. 华南师范大学环境研究院环境理论化学教育部重点实验室, 广州 510006
2. Liaoning Provincial Key Laboratory for Urban Ecology, Shenyang 110167, China;
3. Key Laboratory of Clean Combustion for Electricity Generation and Heat-supply Technology of Liaoning Province, Shenyang Institute of Engineering, Shenyang 110136, China;
4. Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110016, China;
5. The Environmental Research Institute, MOE Key Laboratory of Theoretical Chemistry of Environment, South China Normal University, Guangzhou 510006, China
硒是生物体必需元素,鱼类中有40多种硒蛋白,其中谷胱甘肽过氧化物酶(GPX)通过过氧化氢和有机氢过氧化物来保护鱼类组织抵抗过氧化反应,而硒蛋白P(Se1P)在金属解毒方面发挥重要作用[1-2]。然而,硒的必需性和毒性之间的界限非常狭窄[3-5]。当摄食硒浓度在0.15~0.7 mg·kg-1时,鱼体增重,GPX活性最优,可维持生物体机能平衡[6-9];而当摄食浓度在3~ 4 mg·kg-1时,会对鱼类造成不利影响[10-12]。已有野外观测研究发现,硒对鱼类的毒害作用包括鱼种群减少、生殖障碍、发育畸形等[13-15]。美国加利福尼亚凯斯特森(Kesterson)湿地硒污染案例显示,农业排水的硒浓度为140~1400 μg·L-1时,会导致鱼类和水禽的畸变和死亡[16]。水生态系统中的硒主要以氧化态的无机阴离子形式存在:亚硒酸盐[Se(Ⅳ)]和硒酸盐[Se(Ⅵ)][17]。随着硒环境污染(包括富硒土壤灌溉排水、炼油厂释放、燃煤电厂飞灰等)不断增加[18],硒的毒性影响研究得到越来越多的关注[19]。
硒的毒性机制研究主要集中在两方面:一方面归因于硒和硫的化学性质相似,硒替换硫元素,从而减少巯基对细胞的氧化影响;另一方面是硒在蛋白合成中对氨基酸的替换作用[20]。硒暴露对生物体氧化胁迫的生化指标包括谷胱甘肽过氧化物酶(GSH-Px)、脂质过氧化、过氧化氢酶(CAT)、超氧化物歧化酶(SOD)等[21-24]。机体内抗氧化系统是防御污染胁迫的第一道屏障,当鱼体所处的水环境受到污染时,水体中污染物通过呼吸或摄食进入鱼体,之后通过血液循环进入其他组织器官,进而对鱼体产生影响[21-22]。CAT作为重要的抗氧化因子,是表征生物体健康变化的良好生物标记[23]。同时,总抗氧化能力(T-AOC)代表机体在受到胁迫后的防御能力,是用于衡量机体抗氧化系统功能状况的综合性指标[24]。还有研究表明,在水生生物的生长、发育以及繁殖过程中,Na+/ K+-ATPase对渗透调节和离子调控起着重要的作用,而且Na+/K+-ATPase对金属离子的暴露非常敏感[25-29]。以前有关硒的研究,大部分集中在硒与其他污染物的相互作用,探讨硒对于生物体暴露污染物(如汞、铅、铬、镉)的保护作用[30-32],或者是集中在硒对生物体繁殖(如胚胎发育、产卵量)的影响研究[6, 33],近年来,将生物机体抗氧化因子作为反映污染物对鱼类等机体损伤程度的生态毒理指标已被国内外学者广泛应用[12, 21, 34-36]。斑马鱼(Danio rerio)体型小、繁殖周期短、繁殖力强,短时间易获得大量个体,对毒物敏感,是毒理学试验理想的模式生物[37]。鉴于水相暴露硒对斑马鱼的影响研究相对较少,且缺乏Se(Ⅳ)和Se(Ⅵ)毒性的对比研究,本研究以斑马鱼为实验生物,研究水相暴露Se(Ⅳ)和Se(Ⅵ)在斑马鱼脑、鳃、肝、肠和肌肉组织中的硒累积情况,以及对过氧化氢酶(CAT)、总抗氧化能力(T-AOC)和Na+/K+-ATPase活性的影响,以期为硒的生态毒理效应及生态风险评估提供理论依据。
1 材料与方法 1.1 试验材料 1.1.1 试验动物供试生物斑马鱼(Danio rerio)购于沈阳市水产批发市场,为4个月大的成鱼,属于鲤科短鱼丹属淡水鱼,是国际标准化组织推荐的模式生物。将斑马鱼置于实验室水族箱(40 cm×35 cm×30 cm,长×宽×高)中驯养两周,使其适应实验室环境,驯养期间斑马鱼活动均正常,无病,死亡率低于3%。
1.1.2 主要试剂亚硒酸钠[Se(Ⅳ)]和硒酸钠[Se(Ⅵ)]均购自Sigma公司,纯度≥ 99.0%,色谱级浓硝酸(70%)购自阿拉丁,过氧化氢酶(CAT)、总抗氧化能力(T-AOC)和Na+/K+-ATPase测定试剂盒均购自南京建成生物工程研究所。
试验用水为中等硬度水(MHR),硬水的配方为:NaHCO3 192 mg·L-1、CaSO4 94.9 mg·L-1、MgSO4·7H2O 246 mg·L-1和KCl 8 mg·L-1。所用试剂均为分析纯,购自国药集团化学试剂有限公司。试验中各种溶液的配制用水均为去离子水。
1.2 试验方法 1.2.1 试验设计选取大小相近且健康的斑马鱼(平均体长3.08 cm,平均体重158.36 mg),前期研究结果表明Se(Ⅳ)和Se(Ⅵ)对斑马鱼的96 h-LC50分别为3.95 mg·L-1和40.8 mg·L-1,Se(Ⅳ)和Se(Ⅵ)浓度分别设置为1/8的96 h-LC50,即500 μg·L-1和5000 μg·L-1。试验共设置3组,空白对照组、Se(Ⅳ)处理组(500 μg·L-1)和Se(Ⅵ)处理组(5000 μg·L-1)。每组3个平行,鱼缸的长×宽×高为35 cm×25 cm×20 cm,暴露液体积为10 L,每缸随机放置斑马鱼10尾。试验期间每2 d换一次水,暴露时间为28 d[38]。每日投喂基础饵料2次,连续曝气。暴露期间各鱼缸均无斑马鱼死亡记录。光暗周期比为16 h:8 h,水温(24±1)℃,pH值6.8,溶解氧8.0~8.2 mg·L-1,硬度以CaCO3计为(250 ± 25)mg·L-1。
1.2.2 样品采集暴露时间结束后,每缸随机选取斑马鱼擦干表面,解剖,迅速取出斑马鱼的脑、鳃、肝、肠和肌肉组织样品,用冰生理盐水(0.75% NaCl和0.03% KCl)清洗血液,滤纸吸干,称取适量置于试管中,置-20 ℃冰箱中保存备用。
1.2.3 样品测定斑马鱼组织样品Se元素的测定参照GB 5009.93 —2010方法[39],利用双道原子荧光光度计(AFS- 9700A)测定。整个测定过程中用标准物质(GBW10024-扇贝组织,计量科学研究院)进行分析质量控制,回收率为98%。
过氧化氢酶(CAT)、总抗氧化能力(T-AOC)和Na+/K+-ATPase的测定按照试剂盒说明书进行[40-44]。将各组织样品按1:9质量体积比(m:V)加入预冷生理盐水,冰浴匀浆,离心取上清备用。蛋白质含量的测定采用Bradford法[45]。CAT活性单位定义为每毫克组织蛋白酶分解1 μmol H2O2的量为1个活性单位(U· mg-1 protein)。T-AOC定义为在30 ℃条件下,每分钟每毫克组织蛋白使反应体系的吸光度值每增加0.01时,为1个总抗氧化能力单位(U·mg-1 protein)。Na+/ K+-ATPase活性单位定义为每小时每毫克蛋白分解ATP产生1 μmol无机磷量为1个活性单位(μmol Pi· h-1·mg-1 protein)。试验中每个指标3个平行,将测定的平均值作为最终结果。
1.3 统计分析所得的数据以平均值±标准差(Mean±SD)表示,用SPSS 17.0统计软件进行单因素方差分析(OneWay ANOVA),P < 0.05表示差异显著。
2 结果与分析 2.1 斑马鱼各组织中硒累积含量斑马鱼各组织中硒累积含量如图 1所示。与对照组相比,Se(Ⅳ)和Se(Ⅵ)处理组斑马鱼肝组织中硒累积量最高,分别为(9.62±0.82)μg·g-1和(7.86±0.27)μg·g-1。Se(Ⅳ)与Se(Ⅵ)处理组相比,斑马鱼脑、肠和肌肉组织中硒累积含量差异不显著(P>0.05),肝和鳃组织中硒累积含量显著升高(P < 0.05),其中肝组织中硒累积量显著升高22.4%。

|
不同字母表示处理间差异显著(P<0.05)。下同 Different letters indicate significant difference among the treatments(P<0.05). The same below 图 1 对照组和硒处理组中斑马鱼脑、鳃、肝、肠、肌肉组织中总硒含量(n=3) Figure 1 Se levels in brain, gill, liver, intestine and muscle of control and Se-exposed zebrafish groups(n=3) |
斑马鱼各组织中CAT活性如图 2所示。与对照组相比,Se(Ⅳ)和Se(Ⅵ)处理组,斑马鱼肌肉组织中CAT活性分别显著增加33.3%和7.6%(P < 0.05)。肠组织中CAT活性分别显著降低22.5%和19.7%(P < 0.05)。Se(Ⅳ)与Se(Ⅵ)处理组相比,斑马鱼脑、鳃和肠组织CAT活性差异不显著(P>0.05),肝和肌肉组织中CAT活性差异显著(P < 0.05),其中肝组织中CAT活性显著下降13%。
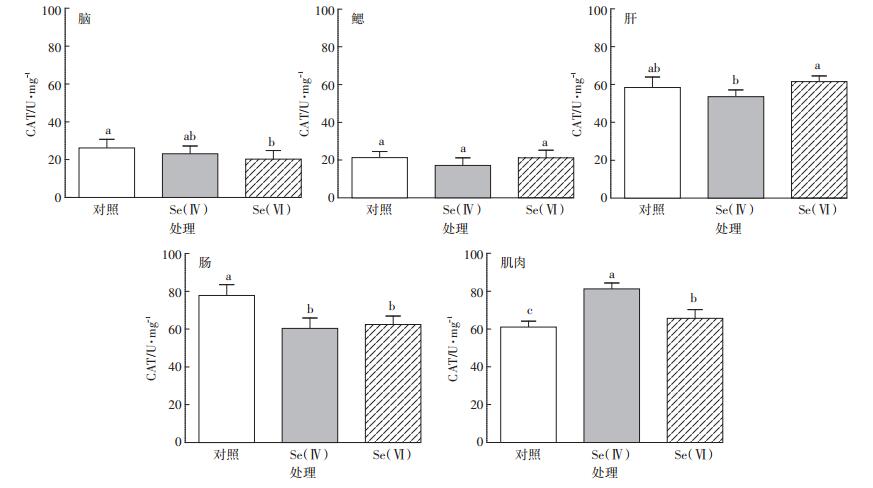
|
图 2 对照组和硒处理组中斑马鱼脑、鳃、肝、肠、肌肉组织中CAT活性(n=3) Figure 2 CAT activity in brain, gill, liver, intestine and muscle of control and Se-exposed zebrafish groups(n=3) |
斑马鱼各组织中T-AOC活性如图 3所示。与对照组相比,Se(Ⅳ)处理组斑马鱼鳃和肠组织以及Se(Ⅵ)处理组斑马鱼鳃、肝和肠组织中T-AOC活性均显著下降(P < 0.05),而Se(Ⅳ)处理组斑马鱼脑组织中T-AOC活性显著升高(P < 0.05)。
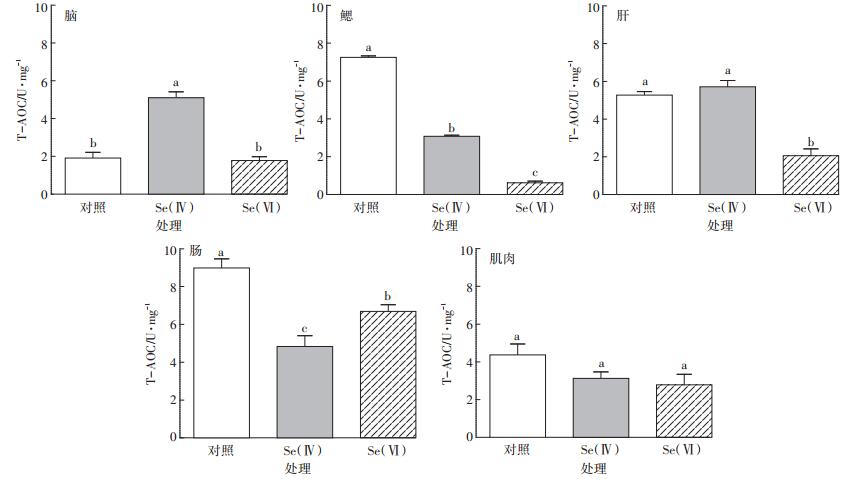
|
图 3 对照组和硒处理组中斑马鱼脑、鳃、肝、肠、肌肉组织中T-AOC活性(n=3) Figure 3 T-AOC activity in brain, gill, liver, intestine and muscle of control and Se-exposed zebrafish groups(n=3) |
Se(Ⅳ)与Se(Ⅵ)处理组相比,斑马鱼肌肉组织中T-AOC活性差异不显著(P>0.05),而脑、鳃、肝和肠组织中T-AOC活性差异显著(P < 0.05),其中鳃和肝组织中T-AOC活性分别显著升高4.0倍和1.8倍。
2.4 斑马鱼各组织中Na+/K+-ATPase活性斑马鱼各组织中Na+/K+-ATPase活性如图 4所示。与对照组相比,Se(Ⅳ)处理组斑马鱼鳃和肝组织以及Se(Ⅵ)处理组斑马鱼脑、鳃、肝、肠和肌肉组织中Na+/K+-ATPase活性均显著降低(P < 0.05)。Se(Ⅳ)处理组斑马鱼肌肉组织中Na+/K+-ATPase活性显著升高(P < 0.05)。
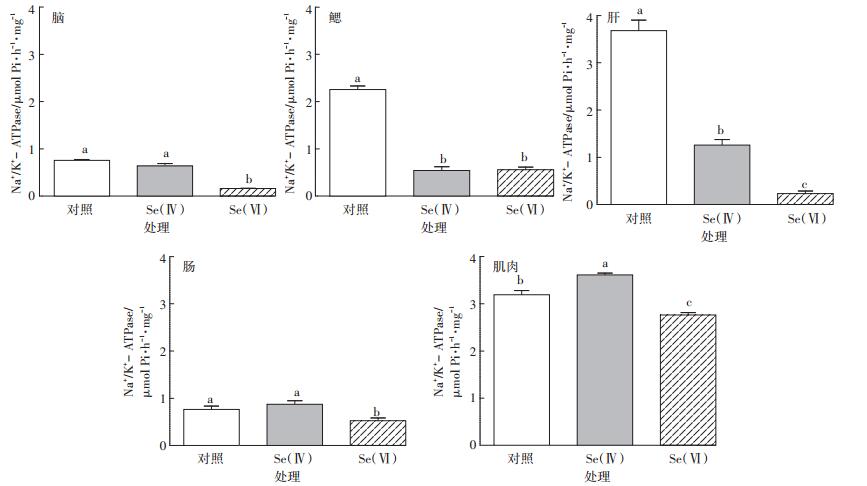
|
图 4 对照组和硒处理组中斑马鱼脑、鳃、肝、肠、肌肉组织中Na+/K+-ATPase活性(n=3) Figure 4 Na+/K+-ATPase activity in brain, gill, liver, intestine and muscle of control and Se-exposed zebrafish groups(n=3) |
Se(Ⅳ)与Se(Ⅵ)处理组相比,斑马鱼鳃组织中Na+/K+-ATPase活性差异不显著(P>0.05),而脑、肝、肠和肌肉组织中Na+/K+-ATPase活性差异显著(P < 0.05),其中Se(Ⅵ)处理组斑马鱼肝组织中Na+/K+- ATPase活性降低更明显。
3 讨论 3.1 硒对斑马鱼各组织中金属元素含量的影响本研究中,Se(Ⅳ)和Se(Ⅵ)处理组斑马鱼肝组织中硒累积量最高,分别为(9.62±0.82)μg·g-1和(7.86 ± 0.27)μg·g-1。类似的硒暴露鱼的研究表明,10 μg·L-1Se(Ⅳ)暴露30 d后,蓝鳃太阳鱼的肝组织中硒累积量为3.0~6.6 μg·g-1,黑鲈鱼的肝组织中硒累积量为5.0~ 7.4 μg·g-1[46]。200 μg·L-1Se(Ⅳ)暴露14 d和28 d后,红鲷鱼的肝组织中硒累积量分别为13 μg·g-1和18 μg·g-1[38]。肝脏是鱼的消化和代谢器官,对重金属的代谢起着非常重要的作用。因为肝脏的解毒作用,使其成为鱼组织中污染物累积的主要部位[47-49]。
本研究中Se(Ⅳ)比Se(Ⅵ)更容易在斑马鱼肝和鳃组织中累积,且累积得更快。类似的关于硒暴露水生生物的研究表明,4 μg·L-1 Se(Ⅳ)暴露10 d后,摇蚊幼虫的Se(Ⅳ)累积量达14 μg·g-1,然而同样浓度的Se(Ⅵ)暴露的累积量可以忽略不计[50]。15 μg·L-1 Se(Ⅳ)和Se(Ⅵ)暴露14 d后,Se(Ⅳ)暴露组寡毛纲带丝蚓的硒累积含量是Se(Ⅵ)暴露组的2倍[25]。Se(Ⅳ)和Se(Ⅵ)处理组斑马鱼组织中硒累积量的差异,可能是由于Se(Ⅳ)和Se(Ⅵ)的化学性质不同,硒酸盐进入细胞是通过和硫酸根一样的离子通道[51]。Se(Ⅳ)和Se(Ⅵ)被鱼吸收,首先在鱼鳃中进行累积,然后分布到鱼的各组织中[46]。然而,Se(Ⅳ)比Se(Ⅵ)具有更强的化学活性,在一些情况下,Se(Ⅳ)还没有被吸收就会被快速地还原成硒化物,从而更稳定地合成到动物组织中[52],这可以部分解释Se(Ⅳ)和Se(Ⅵ)在鱼组织中累积量的不同。
3.2 硒对斑马鱼各组织中CAT活性的影响酶类是生物体内所有生化反应的中间体,任何生化反应都可以通过酶活性来反映生化反应的变化。我们通过测定斑马鱼各组织的酶活性来了解水相暴露硒对鱼体机能的影响程度。
CAT作为生物体内主要的抗氧化酶之一,正常情况下会联合SOD酶清除机体内的活性自由基,使自由基的产生与清除处于动态平衡,机体维持低而有效的自由基浓度,保护机体免受自由基伤害[53]。然而,环境污染胁迫会导致机体自由基产生过多,抗氧化系统受到损伤,使抗氧化酶的活性改变,脂质过氧化损伤,进而导致各种病理生理过程发生[54-55]。
本研究中,硒处理组与对照组相比,斑马鱼肌肉组织中CAT均显著增加,说明硒累积可以诱导CAT活性的增加,这可能是由于累积在鱼体内的硒,激发了大量的超氧化物阴离子自由基,CAT参与解毒作用所致[10, 25]。同样的,Misra等[23]研究表明,虹鳟鱼肝组织暴露有机硒24 h后,CAT活性显著升高。Weekley等[56]关于有机硒的作用机制研究也表明这一点。本研究中,硒处理组与对照组相比,斑马鱼肠组织中CAT均显著降低,而且Se(Ⅳ)与Se(Ⅵ)处理组相比,斑马鱼肝组织中CAT活性显著下降,表明过量的硒累积可能会引发大量自由基的产生,从而压制抗氧化酶(如CAT、SOD等)的清除能力,进而导致ROS的增加,CAT活性下降[10]。Pacini等[57]研究表明,白鲟鱼暴露有机硒30 d,肝组织中CAT的活性随着硒累积的增加显著降低。本研究中,Se(Ⅳ)处理组斑马鱼肝组织的硒累积量显著高于Se(Ⅵ)处理组,同时,硒处理组斑马鱼肝组织中硒的累积量显著高于肌肉组织,这些也从侧面证实了本研究中硒累积导致的CAT活性变化的情况。
3.3 硒对斑马鱼各组织中T-AOC活性的影响T-AOC是近年研究发现的用于衡量机体抗氧化酶系统和非酶促系统功能状况的综合性指标,它的大小可代表和反映机体抗氧化酶系统和非酶系统对外来刺激的代偿能力以及机体自由基代谢的状态[58-61]。王春凤[35]研究表明暴露高汞组染毒5 d时,剑尾鱼鳃和肝组织T-AOC分别降低了39%和30%,而高汞加硒组的T-AOC有明显升高的趋势,表明机体硒可以通过与汞离子结合降低汞离子对机体的毒害作用,提高机体的抗氧化能力。本研究中,与对照组相比,Se(Ⅳ)处理组斑马鱼鳃和肠组织以及Se(Ⅵ)处理组斑马鱼鳃、肝和肠组织中T-AOC活性均显著下降。表明Se可以通过破坏机体细胞膜的完整性和降低酶的活性来降低机体的抗氧化能力[35]。本研究硒处理组斑马鱼肠组织中CAT活性显著降低也能很好地佐证这一点。本研究中,Se(Ⅳ)与Se(Ⅵ)处理组相比,斑马鱼鳃和肝组织中T-AOC活性显著升高,Se(Ⅳ)处理组斑马鱼脑组织中T-AOC活性显著高于对照组。类似研究报道表明,无机硒暴露仔猪或大鼠导致肝组织中T-AOC活性增加[62-63]。随着硒累积量的增加,T-AOC活性显著升高,可能是由于硒累积造成GSH-Px和SOD活性升高引起的[64-65]。
3.4 硒对斑马鱼各组织中Na+/K+-ATPase活性的影响Na+/K+-ATPase主要负责通过细胞质膜共轭Na+和K+的流入和排出。Na+/K+-ATPase酶长时间受到抑制是一种毒性影响,会导致离子调控能力下降、形态学改变、心脏停搏,最终导致生物体死亡[35]。Na+/K+- ATPase是多种毒物作用的靶点,金属与有机物均被认为是Na+/K+-ATPase的强抑制剂。本研究中,硒处理组与对照组相比,Se(Ⅳ)处理组斑马鱼鳃和肝组织以及Se(Ⅵ)处理组斑马鱼脑、鳃、肝、肠和肌肉组织中Na+/K+-ATPase活性均显著下降,说明硒在斑马鱼组织中的累积,导致组织能量得不到充足的供应,代谢活动受到干扰,抵抗力降低,加速了组织的衰老及死亡[25]。这与Evans等[66]的研究一致,硒暴露水生寡毛纲动物后,Na+/K+-ATPase活性显著降低,而这种酶的持续抑制,会降低离子调控能力、改变生物体形态、心脏停搏,最终导致机体死亡。也有文献研究表明,Se(Ⅳ)和有机硒的暴露情况下,Na+/K+-ATPase酶活性的变化有所不同,说明不同形态的硒可能对Na+/ K+-ATPase的活性变化产生不同的影响,氧化态的硒(如+4价或+2价的硒)具有抑制Na+/K+-ATPase活性的能力,而0价或- 2价的硒不具有抑制Na+/K+- ATPase活性的能力[67],这也从侧面解释了本文中Se(Ⅵ)比Se(Ⅳ)处理组斑马鱼肝组织中Na+/K+- ATPase活性下降更明显这一现象,可能是因为+6价的硒比+4价的硒具有更强的抑制Na+/K+-ATPase酶活性的能力。另外,本研究中Se(Ⅳ)处理组与对照组相比,斑马鱼肌肉组织中Na+/K+-ATPase活性显著升高,Se(Ⅵ)处理组斑马鱼肌肉组织中Na+/K+-ATPase活性显著降低。我们发现,Se(Ⅳ)处理组肌肉组织中硒的累积量显著高于对照组,Se(Ⅵ)处理组肌肉组织中硒的累积量与对照相比差异不显著。说明硒的形态与硒的累积量相比,对Na+/K+-ATPase活性抑制的影响可能占主导地位。此外,也有研究表明,虹鳟鱼幼鱼在水相Se(Ⅵ)暴露情况下,不论是急性暴露3.6 mg·L-1 4 d还是亚急性毒性暴露0.36 mg·L-1 30 d,均没有检测到鳃组织中Na+/K+-ATPase活性的变化[25]。这可能是因为不同的暴露浓度和暴露时间情况下,不同的水生生物组织中Na+/K+-ATPase活性变化存在差异所致。
Na+/K+-ATPase酶抑制会破坏鱼的细胞膜结构,损害质膜或线粒体膜,最终导致生物体自身代谢活动受到抑制。ATPase担负着各种离子的跨细胞膜运输的任务,在维持生物电现象、保持盐分、水分平衡和非电解质运输中具有非常重要的作用。很多研究报道认为ATPase能被重金属离子抑制,是因为这些重金属可以通过与膜上蛋白质的结合位点(如含巯基结构或氧基结构的基团)结合后,引起蛋白质空间构象发生变化,这种变化阻止了底物与蛋白质的结合,从而抑制了正常酶的活性[68]。这符合Na+/K+-ATPase的作用机理-构象变化假说[69],即Na+/K+-ATPase本身的构象变化调节着Na+和K+的运输。硒累积导致Na+/K+- ATPase酶受抑制的机制研究相对较少,硒的这种毒性作用可能与硒的化学性质和硫相似有关,可以在合成蛋白的时候将硫替换[17],引起酶的构象发生变化,从而使酶活性中心的构型和低介电区域发生变化,进而影响底物与酶的靠近及定向,底物分子中的敏感性发生变化,最终影响Na+/K+-ATPase活性。
4 结论(1)水相暴露硒导致斑马鱼各组织呈现不同程度的硒累积,硒处理组斑马鱼肝组织中硒累积量最大,Se(Ⅳ)比Se(Ⅵ)更容易在斑马鱼肝和鳃组织中累积,具有更高的生物可利用性。
(2)硒累积导致模式生物斑马鱼组织发生不同程度的氧化胁迫。
(3)CAT、T-AOC和Na+/K+-ATPase生化指标的研究,为进一步从抗氧化系统的角度研究硒暴露对鱼类等水生生物的毒性作用机制奠定了基础。
| [1] |
Roman M, Jitaru P, Barbante C. Selenium biochemistry and its role for human health[J]. Metallomics, 2014, 6(1): 25-54. DOI:10.1039/C3MT00185G |
| [2] |
Allan C B, Lacourciere G M, Stadtman T C. Responsiveness of selenoproteins to dietary selenium[J]. Annual Review of Nutrition, 1999, 19: 1-16. DOI:10.1146/annurev.nutr.19.1.1 |
| [3] |
Levander O A. A global view of human selenium nutrition[J]. Annual Review of Nutrition, 1987, 7: 227-250. DOI:10.1146/annurev.nu.07.070187.001303 |
| [4] |
Ellis D R, Salt D E. Plants, selenium and human health[J]. Current Opinion in Plant Biology, 2003, 6(3): 273-279. DOI:10.1016/S1369-5266(03)00030-X |
| [5] |
Sager M. Selenium in agriculture, food and nutrition[J]. Pure and Applied Chemistry, 2006, 78: 111-133. DOI:10.1351/pac200678010111 |
| [6] |
Ma Y, Wu M, Li D, et al. Embryonic developmental toxicity of selenite in zebrafish (Danio rerio) and prevention with folic acid[J]. Food and Chemical Toxicology, 2012, 50(8): 2854-2863. DOI:10.1016/j.fct.2012.04.037 |
| [7] |
Kouba A, Velíšek J, Stará A, et al. Supplementation with sodium selenite and selenium-enriched microalgae biomass show varying effects on blood enzymes activities, antioxidant response, and accumulation in common barbel(Barbus barbus)[J]. Biomed Research International, 2014(1): 408270. |
| [8] |
Rayman M P. Selenium in cancer prevention:A review of the evidence and mechanism of action[J]. Proceedings of the Nutrition Society, 2005, 64(4): 527-542. |
| [9] |
Lemly A D. Aquatic selenium pollution is a global environmental safety issue[J]. Ecotoxicology and Environmental Safety, 2004, 59(1): 44-56. |
| [10] |
Valko M, Morris H, Cronin M T. Metals, toxicity and oxidative stress[J]. Current Medicinal Chemistry, 2005, 12(10): 1161-1208. DOI:10.2174/0929867053764635 |
| [11] |
Lemly A D. A teratogenic deformity index for evaluating impacts of selenium on fish populations[J]. Ecotoxicology and Environmental Safety, 1997, 37(3): 259-266. |
| [12] |
Xie L T, Wu X, Chen H X, et al. A low level of dietary selenium has both beneficial and toxic effects and is protective against Cd-toxicity in the least killifish Heterandria formosa[J]. Chemosphere, 2016, 161: 358-364. DOI:10.1016/j.chemosphere.2016.07.035 |
| [13] |
Hamilton S J. Review of selenium toxicity in the aquatic food chain[J]. Science of the Total Environment, 2004, 326(1/2/3): 1-31. |
| [14] |
Hoffman D J. Role of selenium toxicity and oxidative stress in aquatic birds[J]. Aquatic Toxicology, 2002, 57(1/2): 11-26. |
| [15] |
Ohlendorf H M. The birds of Kesterson Reservoir:A historical perspective[J]. Aquatic Toxicology, 2002, 57(1/2): 1-10. |
| [16] |
US Geological Survey. Areal distribution of selenium and other inorganic constituents in shallow ground water of the San Luis Drain Service Area, San Joaquin Valley, California: A preliminary study[R]. California: Water-Resources Investigations Report, 1984.
|
| [17] |
Chapman P M, Adams W J, Brooks M, et al. Ecological assessment of selenium in the aquatic environment[M]. Florida: CRC Press, 2010.
|
| [18] |
Kupsco A, Schlenk D. Mechanisms of selenomethionine developmental toxicity and the impacts of combined hypersaline conditions on Japanese medaka(Oryzias latipes)[J]. Environmental Science & Technology, 2014, 48(12): 7062-7068. |
| [19] |
李保民. 环境中的硒-生物效应、形态、浓度水平及人体摄入标准[J]. 环境保护科学, 1988, 14(1): 66-72. LI Bao-min. Selenium in the environment:Biological effect, forms, concentration level and human consumption standard[J]. Environmental Protection Science, 1988, 14(1): 66-72. |
| [20] |
Maier K J, Knight A W. Ecotoxicology of selenium in freshwater systems[J]. Reviews of Environmental Contamination and Toxicology, 1994, 134: 31-48. |
| [21] |
Miller L L, Wang F, Palace V P, et al. Effects of acute and subchronic exposures to waterborne selenite on the physiological stress response and oxidative stress indicators in juvenile rainbow trout[J]. Aquatic Toxicology, 2007, 83(4): 263-271. |
| [22] |
Orun I, Talas Z S, Ozdemir I, et al. Antioxidative role of selenium on some tissues of(Cd2+, Cr3+)-induced rainbow trout[J]. Ecotoxicology and Environmental Safety, 2008, 71(1): 71-75. |
| [23] |
Misra S, Niyogi S. Selenite causes cytotoxicity in rainbow trout(Oncorhynchus mykiss)hepatocytes by inducing oxidative stress[J]. Toxicology in Vitro, 2009, 23(7): 1249-1258. DOI:10.1016/j.tiv.2009.07.031 |
| [24] |
Abbas H H H, Authman M M N. Effects of accumulated selenium on some physiological parameters and oxidative stress indicators in Tilapia fish(Oreochromis spp.)[J]. American-Eurasian Journal of Agricultural and Environmental Science, 2009, 5(2): 219-225. |
| [25] |
Xie L T, Wu X, Chen H X, et al. The bioaccumulation and effects of selenium in the oligochaete Lumbriculus variegatus via dissolved and dietary exposure routes[J]. Aquatic Toxicology, 2016, 178: 1-7. DOI:10.1016/j.aquatox.2016.07.008 |
| [26] |
Jagoe C H, Shaw-Allen P L, Brundage S. Gill Na+, K+-ATPase activity in largemouth bass (Micropterus salmoides)from three reservoirs with different levels of mercury contamination[J]. Aquatic Toxicology, 1996, 36(3): 161-176. |
| [27] |
Morgan I J, Henry R P, Wood C M. The mechanism of acute silver nitrate toxicity in freshwater rainbow trout (Oncorhynchus mykiss) is inhibition of gill Na+ and Cl-transport[J]. Aquatic Toxicology, 1997, 38(96): 145-163. |
| [28] |
贾秀英, 陈志伟. 铜、镉对鲫鱼组织Na+-K+-ATPase酶活性的影响[J]. 科技通报, 2003, 19(1): 50-53. JIA Xiu-ying, CHEN Zhi-wei. Effects of copper and cadmium on Na+-K+-ATPase activity in tissues of Carassius auratus[J]. Bulletin of Science and Technology, 2003, 19(1): 50-53. DOI:10.3969/j.issn.1001-7119.2003.01.012 |
| [29] |
袁锦芳, 陈叙龙, 张毓琪. 环境因素对海洋动物Na+-K+-ATPase的影响概述[J]. 海洋环境科学, 1999, 18(3): 75-79. YUAN Jin-fang, CHEN Xu-long, ZHANG Yu-qi. Effects of the environmental factors on Na+-K+-ATPase of marine animals[J]. Marine Environmental Science, 1999, 18(3): 75-79. DOI:10.3969/j.issn.1007-6336.1999.03.015 |
| [30] |
Ates B, Orun I, Talas Z S, et al. Effects of sodium selenite on some biochemical and hematological parameters of rainbow trout (Oncorhynchus mykiss Walbaum, 1792) exposed to Pb2+ and Cu2+[J]. Fish Physiology and Biochemistry, 2008, 34(1): 53-59. |
| [31] |
Ralston N V, Raymond L J. Dietary selenium's protective effects against methylmercury toxicity[J]. Toxicology, 2010, 278(1): 112-123. DOI:10.1016/j.tox.2010.06.004 |
| [32] |
Bjerregaard P, Fjordside S, Hansen M G, et al. Dietary selenium reduces retention of methylmercury in freshwater fish[J]. Environmental Science and Technology, 2011, 45(22): 9793-9798. DOI:10.1021/es202565g |
| [33] |
U.S. Geological Survey. Bioaccumulation and toxicity of selenium during a life-cycle exposure with desert pupfish (Cyprinodon macularius)[R]. Columbia: U. S. Geological Survey Scientific Investigations Report, 2012.
|
| [34] |
Manduzio H, Monsinjon T, Rocher B, et al. Characterization of an inducible isoform of the Cu/Zn superoxide dismutase in the blue mussel Mytilus edulis[J]. Aquatic Toxicology, 2003, 64(1): 73-83. |
| [35] |
王春凤.硒对汞致剑尾鱼抗氧化系统的毒害和生理损伤的拮抗作用[D].广州: 华南师范大学, 2004. WANG Chun-feng. The antagonistic action of selenium on the harm of antioxidant system and physiological damage in swordtail fish (Xiphophorus helleri) under the exposure of mercury[D]. Guangzhou: South China Normal University, 2004. http://cdmd.cnki.com.cn/Article/CDMD-10574-2004074145.htm |
| [36] |
Ma S S, Zhou Y, Chen H X, et al. Selenium accumulation and the effects on the liver of topmouth gudgeon Pseudorasbora parva exposed to dissolved inorganic selenium[J]. Ecotoxicology and Environmental Safety, 2018, 160: 240-248. DOI:10.1016/j.ecoenv.2018.05.047 |
| [37] |
Oberemm A. The use of a refined zebrafish embryo bioassay for the assessment of aquatic toxicity[J]. Laboratory Animals, 2000, 29(7): 32-40. |
| [38] |
Kim J H, Kang J C. The selenium accumulation and its effect on growth, and haematological parameters in red sea bream, Pagrus major, exposed to waterborne selenium[J]. Ecotoxicology and Environmental Safety, 2014, 104: 96-102. DOI:10.1016/j.ecoenv.2014.02.010 |
| [39] |
Yu D, Liang D, Lei L, et al. Selenium geochemical distribution in the environment and predicted human daily dietary intake in northeastern Qinghai, China[J]. Environmental Science and Pollution Research(international), 2015, 22(15): 1-12. |
| [40] |
Zhang X L, An L J, Bao Y M, et al. D-galactose administration induces memory loss and energy metabolism disturbance in mice:Protective effects of catalpol[J]. Food and Chemical Toxicology, 2008, 46(8): 2888-2894. DOI:10.1016/j.fct.2008.05.032 |
| [41] |
Deng X, Wu F, Liu Z, et al. The splenic toxicity of water soluble multi-walled carbon nanotubes in mice[J]. Carbon, 2009, 47(6): 1421-1428. DOI:10.1016/j.carbon.2008.12.032 |
| [42] |
Hou H, Li B, Zhao X, et al. The effect of pacific cod (Gadus macrocephalus) skin gelatin polypeptides on UV radiation-induced skin photoaging in ICR mice[J]. Food Chemistry, 2009, 115(3): 945-950. DOI:10.1016/j.foodchem.2009.01.015 |
| [43] |
Sun D Q, Li A W, Li J, et al. Changes of lipid peroxidation in carbon disulfide-treated rat nerve tissues and serum[J]. Chemico-Biological Interactions, 2009, 179(2/3): 110-117. |
| [44] |
Anjum S A, Tanveer M, Hussain S, et al. Cadmium toxicity in maize (Zea mays L.):Consequences on antioxidative systems, reactive oxygen species and cadmium accumulation[J]. Environmental Science and Pollution Research, 2015, 22(21): 17022-17030. DOI:10.1007/s11356-015-4882-z |
| [45] |
Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding[J]. Analytical Biochemistry, 1976, 72(1/2): 248-254. |
| [46] |
Lemly A D. Response of juvenile centrarchids to sublethal concentrations of waterborne selenium. 1. Uptake, tissue distribution, and retention[J]. Aquatic Toxicology, 1982, 2(4): 235-252. DOI:10.1016/0166-445X(82)90027-3 |
| [47] |
Lemly A D. Symptoms and implications of selenium toxicity in fish:the Belews Lake case example[J]. Aquatic Toxicology, 2002, 57: 39-49. DOI:10.1016/S0166-445X(01)00264-8 |
| [48] |
Li L X, Chen H X, Bi R, et al. Bioaccumulation, subcellular distribution, and acute effects of chromium in Japanese medaka(Oryzias latipes)[J]. Environmental Toxicology and Chemistry, 2015, 34(11): 2611-2617. DOI:10.1002/etc.3112 |
| [49] |
孟凡信.威海沿岸几种海洋鱼类重金属含量的研究[D].济南: 山东大学, 2006. MENG Fan-xin. Research on the heavy metal content of several marine fishes along Weihai coastal area[D]. Jinan: Shandong University, 2006. http://cdmd.cnki.com.cn/Article/CDMD-10422-2006167372.htm |
| [50] |
Franz E D, Wiramanaden C I E, Janz D M, et al. Selenium bioaccumulation and speciation in Chironomus dilutus exposed to water-borne selenate, selenite, or seleno-DL-methionine[J]. Environmental Toxicology and Chemistry, 2011, 30(10): 2292-2299. DOI:10.1002/etc.v30.10 |
| [51] |
Silva J, Williams R. The biological chemistry of the elements:The inorganic chemistry of life[J]. Biochemical Education, 1992, 20: 62-63. |
| [52] |
Sharma V K, McDonald T J, Sohn M, et al. Biogeochemistry of selenium. A review[J]. Environmental Chemistry Letters, 2015, 13(1): 49-58. DOI:10.1007/s10311-014-0487-x |
| [53] |
Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia[J]. Kidney International, 1996, 49(5): 1304-1313. DOI:10.1038/ki.1996.186 |
| [54] |
Daneshvar B, Frandsen H, Autrupand H, et al. P-glutamyl semialdehyde and 2-amino-adipic semialdehyde:Biomarkers of oxidative damage to proteins[J]. Biomarkers, 1997, 2(2): 117-123. |
| [55] |
Kohen R, Nyska A. Oxidation of biological systems:oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification[J]. Toxicologic Pathology, 2002, 30(6): 620-650. DOI:10.1080/01926230290166724 |
| [56] |
Weekley C M, Jeong G, Tierney M E, et al. Selenite-mediated production of superoxide radical anions in A549 cancer cells is accompanied by a selective increase in SOD1 concentration, enhanced apoptosis and Se-Cu bonding[J]. Journal of Biological Inorganic Chemistry, 2014, 19(6): 813-828. DOI:10.1007/s00775-014-1113-x |
| [57] |
Pacini N, Elia A C, Abete M C, et al. Antioxidant response verses selenium accumulation in the liver and kidney of the Siberian sturgeon (Acipenser baeri)[J]. Chemosphere, 2013, 93(10): 2405-2412. DOI:10.1016/j.chemosphere.2013.08.042 |
| [58] |
王丽平, 郑丙辉, 孟伟. 环境污染物对水生生物产生氧化压力的分子生物标志物[J]. 生态学报, 2007, 27(1): 380-388. WANG Li-ping, ZHENG Bing-hui, MENG Wei. Molecular biomarkers in aquatic organisms in relation to the oxidative stress imposed by environmental pollutants[J]. Acta Ecologica Sinica, 2007, 27(1): 380-388. DOI:10.3321/j.issn:1000-0933.2007.01.044 |
| [59] |
Lin C T, Wenchung T, Naiwan H, et al. Characterization, molecular modelling and developmental expression of zebrafish manganese superoxide dismutase[J]. Fish and Shellfish Immunology, 2009, 27(2): 318-324. DOI:10.1016/j.fsi.2009.05.015 |
| [60] |
陈家长, 宋超, 胡庚东, 等. 微囊藻毒素-LR对罗非鱼肝脏活性氧自由基含量及相关抗氧化酶活性的影响[J]. 农业环境科学学报, 2011, 30(8): 1521-1525. CHEN Jia-zhang, SONG Chao, HU Geng-dong, et al. Effects of microcystin-LR on antioxidant enzymes and reactive oxygen species in tilapia fish[J]. Journal of Agro-Environment Science, 2011, 30(8): 1521-1525. |
| [61] |
马莉芳.水体铜对黄河鲤毒性的研究[D].郑州: 河南农业大学, 2013. MA Li-fang. Study on the effects of copper to the toxicity of the yellow river carp[D]. Zhengzhou: Henan Agriculture University, 2013. http://cdmd.cnki.com.cn/Article/CDMD-10466-1014105590.htm |
| [62] |
Chen J, Han J H, Guan W T, et al. Selenium and vitamin E in sow diets:Ⅱ. Effect on selenium status and antioxidant status of the progeny[J]. Animal Feed Science and Technology, 2016, 221: 111-123. DOI:10.1016/j.anifeedsci.2016.08.022 |
| [63] |
Feng P, Wei J R, Zhang Z G. Intervention of selenium on chronic fluorosis-induced injury of blood antioxidant capacity in rats[J]. Biological Trace Element Research, 2011, 144(1/2/3): 1024-1031. |
| [64] |
Feng P, Wei J R, Zhang Z G. Influence of selenium and fluoride on blood antioxidant capacity of rats[J]. Experimental and Toxicologic Pathology, 2012, 64(6): 565-568. DOI:10.1016/j.etp.2010.11.014 |
| [65] |
Kong Y Q, Ding Z L, Zhang Y X, et al. Dietary selenium requirement of juvenile oriental river prawn Macrobrachium nipponense[J]. Aquaculture, 2017, 476: 72-78. DOI:10.1016/j.aquaculture.2017.04.010 |
| [66] |
Evans D H, Piermarini P M, Choe K P, et al. The multifunctional fish gill:Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste[J]. Physiological Reviews, 2005, 85(1): 97-177. DOI:10.1152/physrev.00050.2003 |
| [67] |
Bergad P L, Rathbun W B. Inhibition of Na, K-ATPase by sodium selenite and reversal by glutathione[J]. Current Eye Research, 1986, 5(12): 919-923. DOI:10.3109/02713688608995172 |
| [68] |
Thaker J, Chhaya J, Nuzhat S, et al. Effects of chromium (Ⅵ) on some ion-dependent ATPases in gills, kidney and intestine of a coastal teleost Periophthalmus dipes[J]. Toxicology, 1996, 112(3): 237-244. DOI:10.1016/0300-483X(96)86481-X |
| [69] |
沈同, 王镜岩. 生物化学(下册)[M]. 北京: 人民教育出版社, 1980. SHEN Tong, WANG Jing-yan. Biochemistry(Next volume)[M]. Beijing: The People's Education Press, 1980. |
 2019, Vol. 38
2019, Vol. 38


