2. 农业农村部农膜污染防控重点实验室, 北京 100081;
3. 山东大学化学与化工学院特种功能聚集体材料教育部重点实验室, 山东 250100
2. Key Laboratory for Prevention and Control of Residual Pollution in Agricultural Film, Ministry of Agriculture, Beijing 100081, China;
3. Key Laboratory of Special Functional Aggregated Materials, Ministry of Education, School of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, China
近30年来,地膜覆盖技术在我国得到迅速推广应用,地膜年使用量超过1.45×106 t,覆盖面积超过1.8×107 hm2;该技术应用使大多数农作物普遍增产20%~50%,为保障我国农产品生产安全作出巨大贡献[1-3]。另一方面,地膜的主要成分聚乙烯具有相对分子质量大、分子结晶度高、疏水性强、分子键理化性能稳定、在自然条件下极难完全降解的特性[3-5],使用后大量地膜碎片长期残留于农田土壤中,破坏土壤结构、阻碍土壤水分和养分运移,妨碍农事作业,影响农作物生长发育,降低农产品产量与品质[6-7]。本文依据聚乙烯地膜的分子结构与理化特性,系统综述了国内外对聚乙烯地膜降解过程、机理、微生物等方面的研究进展,为评价不同环境条件下聚乙烯地膜的降解速率,探讨聚乙烯地膜残留污染的综合治理方案提供支撑。
1 聚乙烯地膜降解的概念与特点“降解”意为:①有机化合物分子中的碳原子数目减少,分子量降低;②高分子化合物的大分子分解成较小的分子[8]。文中聚乙烯地膜降解即指构成地膜产品的聚乙烯高分子在光、热、水、氧、机械力等环境因素作用下,发生C-C键氧化断裂,进而引起分子链碳原子数目减少、聚合度及相对分子质量下降,非结晶区及小型结晶区域解聚成亲水性低聚物或小分子,并最终在微生物作用下完全分解为CO2、H2O、CH4、生物质等微生物代谢产物的过程[9-14]。
聚乙烯是由几千至几万个乙烯小分子均聚或与少量1-烯烃共聚生成的线性高分子化合物[4, 15],其降解特性受重复单元、分子链结构、凝聚态结构三个结构层级的共同影响[4, 16],主要包括分子键强度、分子链长度、分子亲/疏水性、分子结晶度等方面[4, 16]。一般而言,分子键强度越大、分子链越长、疏水性越强、结晶度越高,则分子越难降解[4, 16-17]。聚乙烯结构单元(-[CH2-CH2]n-)的C-C、C-H键理化性能稳定,需要较高的能量或作用力才可发生分子键断裂;且相对分子质量大、分子链长、链段结晶度高、疏水性强,难以与生物及化学物质接触或进入微生物体内代谢分解[9, 18-20];因此在自然条件下的降解过程非常缓慢[9-12]。特别要说明的是:在聚乙烯的降解过程中,氢过氧化物和含氧基团的产生是启动自由基链式反应,促进聚乙烯非生物氧化降解的关键;通过检测氢过氧化物或含氧基团的含量及动态变化可以表征聚乙烯地膜的降解活性[12, 21]。此外,通过比较聚乙烯降解生成的CO2或CH4的实际释放量占聚乙烯分子完全分解的理论释放量的比值可推算聚乙烯材料经微生物同化作用实现完全分解的比率,即降解率[11, 14]。Albertsson等[22]通过14C标记聚乙烯材料,跟踪监测聚乙烯薄膜在土壤填埋条件下14CO2释放量,研究发现在土壤中填埋10 a之久的聚乙烯薄膜降解率仅为0.2%~0.5%。Ohtake等[23-25]进一步对土壤中填埋32 a的聚乙烯薄膜和塑料瓶进行表面分子结构与分子量分析,推算60 µm厚度的聚乙烯薄膜在田间土壤填埋条件下实现完全降解大概需要300 a。
另外,聚乙烯材料中,不论二维或三维空间结构,其分子的排列都不是完全均一或规整有序的[12, 26-27]。分子链排列整齐、紧密的区域称为结晶区,较难与生物或化学物质接触反应,不易降解;分子链排列凌乱、疏松的区域称为非结晶区,分子链段柔顺度较高、活动性较大,相对易于在物理能量或化学物质作用下分解断裂[4, 12, 18, 27];可见聚乙烯材料的降解过程具有反应速率不均一的重要特点[4, 17]。在外界因素的不断作用下,从材料表面到材料内部,由聚乙烯分子的非结晶区至小型结晶区域分子链段先后断裂[19, 21, 28],并根据材料降解的严重程度表现出机械强度下降,热力学、光学、电学、密度等理化特性改变,以致龟裂分解等现象[21, 27-28]。Ohtake等[25]对聚乙烯(LDPE)薄膜和塑料瓶的微观结构与数均分子量的研究显示,土壤填埋32 a后材料虽整体基本完整,但表面数均分子量由106下降到103,且出现大量微小孔洞,呈明显腐解现象。
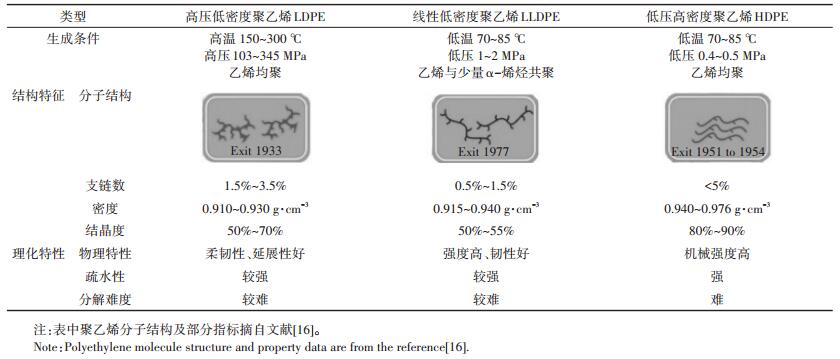
根据生产工艺和理化性质的差异,聚乙烯分子可分为高压低密度聚乙烯(Low-density polyethylene,LDPE)、低压高密度聚乙烯(High-density polyethylene,HDPE)、线性低密度聚乙烯(Linear low-density polyethylene,LLDPE)等不同类型[3, 16]。其中LDPE分子在高温高压条件下生成,反应条件剧烈,生成的分子链分枝数目多、分子排列疏松、结晶度(即聚合物分子链中结晶区所占百分比)相对较低,表现为材料密度小、强度较差,但柔韧性、透光性好,且相对易于降解;HDPE通过催化剂作用在较低温度和压力条件下聚合产生,分枝数目少、分子链排列致密、分子结晶度高,其材料密度大、机械强度高,降解最为缓慢[29-31]。表 1对不同类型聚乙烯分子的主要结构特征[16]与理化特性[16, 29-31]进行了比较。
|
|
表 1 应用于地膜生产的聚乙烯分子结构与性能 Table 1 Structure and properties of polyethylene molecule used in mulch film production |
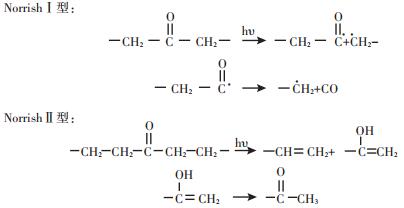
根据聚乙烯高分子降解反应的作用因素与机理,可将其划分为非生物氧化降解与生物氧化降解两个过程[12, 19, 32]。其中非生物氧化降解是指聚乙烯分子链在受到高于其分子间共价键键能的光、热、机械力等非生物因素作用时发生共价键氧化断裂,分解为小分子或低分子量物质的过程[10, 12, 19, 21, 32]。普遍认为其作用机理与小分子碳氢化合物自发催化氧化反应是一致的[12, 19]。具体包括以下步骤:①链引发,聚乙烯分子键在受到机械力或光、热等能量作用时断裂生成高活性自由基;②链增长,高活性自由基在有氧条件下迅速反应生成过氧基或氢过氧基等中间产物;③链转移,含不稳定过氧自由基或氢过氧自由基的化合物发生分子键断裂生成含羰基化合物;④链终止,含羰基化合物通过Norrish Ⅰ型反应生成酰基自由基和烃基自由基,酰基自由基可进一步反应生成一氧化碳、醛、羧酸、酯类等化合物,或者含羰基化合物通过Norrish Ⅱ型反应生成末端乙烯基与甲基酮,甲基酮再经过第二次Norrish Ⅱ型反应产生酮羰基[12, 19]。聚乙烯分子非生物氧化反应过程中生成的羰基等紫外线敏感基团,以及过氧基、氢过氧基等高活性基团易于进一步分解生成自由基,启动聚乙烯分子的自发催化氧化反应,其含量与动态变化能够一定程度反映聚乙烯的降解活性[12, 15](图 1和图 2)。
Albersson等[22]检测发现,经过7、26、42 d紫外照射的LDPE薄膜,在土壤中填埋10 a后失重率分别达到0.3%、0.5%、5.7%,明显高于未经紫外照射的对照薄膜0.2%的失重率,提示紫外辐射能够加快聚乙烯薄膜的降解反应。此外,Jakubowicz等[33]研究发现在25 ℃环境温度下将聚乙烯氧化降解地膜填埋在土壤中4.5 a后,地膜的数均分子质量可下降到10 000以下;而在60 ℃条件下这一过程则只需要180 d。Chiellini等[34]通过分析材料分子量与表面性能等指标,证实随温度升高(55、70 ℃)聚乙烯薄膜分解速度明显加快。为了模拟研究较长时间尺度上聚乙烯地膜的降解情况,Briassoulis等[13]通过人工加热及紫外辐射的方法对聚乙烯残留地膜进行加速老化处理,回填到土壤中观察地膜在自然状态下的分解情况。结果显示,未经人工加速老化处理的聚乙烯薄膜填埋在土壤中8.5年后降解现象不明显,经过高温(50 ℃处理800 h)及紫外辐射(受35~45 W·m-2紫外灯在25 cm距离处照射800 h)的聚乙烯薄膜回填到土壤中8.5 a后被完全分解为直径小于1 mm的塑料微颗粒,且降解过程进一步持续。上述结果说明光、热等环境因素能够明显促进聚乙烯地膜的降解反应[35-36]。
3 聚乙烯地膜生物氧化降解微生物在聚乙烯降解过程中发挥重要作用。早期研究显示,环境中微生物能够对聚乙烯非生物氧化过程产生的小分子物质与数均分子量小于5000的低分子量物质进行快速分解利用[37-38],并伴随自身生长繁殖[39]。近年来研究进一步发现阿氏肠杆菌Enterobacter asburiae、芽胞杆菌Bacillus sp.、波茨坦短芽孢杆菌Brevibacillus borstelensis等微生物能够分别以未经光热预处理且不含促氧化添加剂的数均分子量为28 000甚至191 000的低密度聚乙烯为唯一有机碳源进行分解利用[40-41],为聚乙烯的微生物降解及白色污染的综合治理提供了新的重要途径。
目前从塑料垃圾污染的土壤、污泥、填埋场、堆肥厂、海洋等环境条件中分离得到的可能参与聚乙烯材料降解的微生物有几十种,分别属于细菌中的20个属和真菌中的12个属[14, 41-42, 44, 64]。研究显示这些微生物能够在聚乙烯材料表面附着生长,导致聚乙烯材料在分子结构、分子量、机械性能、材料完整性等方面发生明显改变(表 2)。
|
|
表 2 参与聚乙烯降解过程的主要微生物 Table 2 Primary microorganisms associated with polyethylene biodegradation |
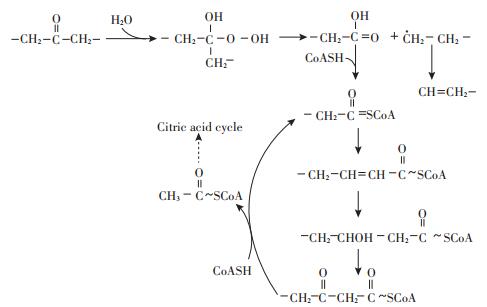
基于聚乙烯降解过程重要微生物的分离鉴定与功能研究,参考具有相似结构特性的线形石蜡分子的生物降解过程,分析推测聚乙烯生物降解包括以下3个步骤[11, 14, 65-66]。①生物腐蚀:微生物能够在细胞表面分泌多糖、蛋白等多聚物构成黏液层,帮助自身抵御不良环境,并聚集空气中的微小物质以促进自身生长繁殖。在聚乙烯降解过程中,该黏液层可以有效降低聚乙烯分子表面疏水性,帮助微生物粘附在材料表面并促进微生物分泌的胞外酶与聚乙烯材料表面分子链段或低分子量物质相互作用。②生物分解:微生物分泌的特定的酶类通过水解、氧化等多重反应将聚乙烯分子链段分解为低分子量寡聚物、二聚体、单体等分子碎片。③生物同化:由聚乙烯分解产生的低分子量物质透过细胞膜进入微生物体内,根据微生物的种类、生长环境等差异分别通过有氧呼吸、厌氧呼吸、发酵等不同途径代谢生成微生物生长繁殖所需的电子、能量(ATP)及构成细胞组分的营养元素等。Kawai等[48]推测在有氧条件下,微生物体内的聚乙烯分子碎片可能通过β-氧化途径分解生成乙酰辅酶A,进一步通过三羧酸循环分解为CO2、H2O与能量物质[12](图 3)。由于经过微生物代谢生成的无机小分子一般均可以进入生物地球化学循环,认为不存在生态毒性[65]。
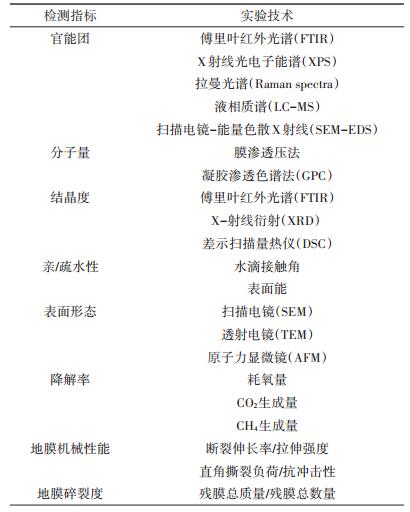
4 聚乙烯地膜降解的研究技术与方法随着近年来聚乙烯地膜白色污染问题的日益凸显,对聚乙烯降解过程的研究受到越来越多的关注,围绕聚乙烯材料的降解机理形成了一系列假说,但仍都需要进行深入系统的研究与论证。针对聚乙烯材料的降解过程,分别从分子官能团、结晶度、相对分子质量、材料理化特性、材料碎裂度等不同层面开展深入系统的研究,对于深入理解聚乙烯材料的降解机理与关键性作用因素具有重要意义[11, 19, 38, 65, 67-69]。表 3中列出了聚乙烯地膜降解过程的主要评价指标与技术方法[11, 14]。
|
|
表 3 聚乙烯地膜降解的常用评价指标与技术方法 Table 3 Common criteria and technical approaches in polyethylene degradation investigation |
本文从聚乙烯材料的分子结构与特性入手,结合国内外研究领域最新进展,围绕聚乙烯地膜在自然环境中的降解过程、降解产物、作用机理、影响因素等方面进行了综合论述,主要包括以下两个方面:
(1)聚乙烯地膜在自然环境中的降解过程。聚乙烯地膜在应用及废弃等过程中出现机械强度下降,光学、热力学性能改变,以及材料龟裂破损等现象都是其降解的具体表现。聚乙烯材料的降解反应包括非生物氧化降解和生物氧化降解两个过程。其中非生物氧化降解过程中,聚乙烯分子链受到高于其分子间共价键键能的光、热、机械力等非生物因素作用并发生共价键断裂,在有氧条件下迅速反应生成过氧基或氢过氧基等不稳定中间产物,进一步反应生成醛、酮、酸、酯、一氧化碳等较稳定含氧化合物。生物氧化降解过程中聚乙烯分子链段或经非生物氧化反应生成的聚乙烯小分子及低分子量物质通过微生物酶解同化,并根据微生物种类及生长环境最终转化为CO2、CH4、H2O、生物质等微生物代谢产物。一般认为,经微生物代谢生成的无机小分子可以进入生物地球化学循环,不存在生态毒性。
(2)聚乙烯地膜的降解速度。聚乙烯地膜的降解速度由自身结构与环境因素共同决定。聚乙烯分子链长、相对分子质量大、链段结晶度高、疏水性强,难以与生物、化学物质接触或进入微生物体内分解代谢;加之组成聚乙烯分子的C-C共价键理化性能稳定,键能强度大,需要较高的能量或作用力才可发生分子键断裂;这些决定了聚乙烯分子难以降解,且降解过程缓慢。研究结果显示土壤中填埋32 a的聚乙烯材料仅表面数均分子量下降并出现大量微小孔洞,但整体仍基本保持完整。Ohtake等[23-25]根据降解速度推算60 µm厚度的聚乙烯薄膜在田间土壤填埋条件下实现完全降解需要大概300 a。影响聚乙烯分子降解的主要限速步骤即分子键断裂生成活性自由基的反应。环境中的光能、热能、机械力、微生物酶促反应等过程可以促进聚乙烯分子键断裂,加快聚乙烯材料降解反应的速度。Briassoulis等[13]研究显示,经高温及紫外辐射处理的聚乙烯残膜在回填到土壤中8.5 a后可被完全分解为直径小于1 mm的塑料微颗粒。
6 展望依据现有降解技术,聚乙烯材料降解过程至少需要几年至几十年的时间。如何加速聚乙烯地膜降解、减少残余地膜对土壤的污染是当前农业生产亟待解决的问题。建议从以下几方面进行研究:
(1)开展聚乙烯产品改良,延缓其机械强度及使其物理性能下降等降解反应现象,提高地膜回收率是减少地膜污染途径之一;
(2)开展聚乙烯降解微生物的筛选鉴定与功能研究,尝试通过微生物的分解同化作用对回收地膜进行集中处理,替代现有残膜填埋、废弃、焚烧等处理方式可有效降低地膜对环境的严重污染;
(3)研究开发新型可生物降解材料替代聚乙烯在地膜中的应用。
| [1] |
Yan C R, He W Q, Neil C T, et al. Plastic-film mulch in Chinese agriculture: Importance and problems[J]. World Agriculture, 2014, 4(2): 32-36. |
| [2] |
严昌荣, 刘恩科, 舒帆, 等. 我国地膜覆盖和残留污染特点与防控技术[J]. 农业资源与环境学报, 2014, 31(2): 95-102. YAN Chang-rong, LIU En-ke, XU Fan, et al. Review of agricultural plastic mulching and its residual pollution and prevention measures in China[J]. Journal of Agricultural Resources and Environment, 2014, 31(2): 95-102. |
| [3] |
严昌荣, 何文清, 刘爽, 等. 中国地膜覆盖及残留污染防控[M]. 北京: 科学出版社, 2015: 279. YAN Chang-rong, HE Wen-qing, LIU Shuang, et al. Application of mulch films and prevention of its residual pollution in China[M]. Beijing: China Science Publication, 2015: 279. |
| [4] |
胡国文, 周智敏, 张凯, 等. 高分子化学与物理学教程[M]. 北京: 科学出版社, 2014. HU Guo-wen, ZHOU Zhi-min, ZHANG Kai, et al. Polymer chemistry and physics[M]. Beijing: Science Press, 2014. |
| [5] |
Kasirajan S, Ngouajio M. Polyethylene and biodegradable mulches for agricultural applications: A review[J]. Agronomy for Sustainable Development, 2012, 32(2): 501-529. |
| [6] |
Liu E K, He W Q, Yan C R. 'White revolution'to'white pollution': Agricultural plastic film mulch in China[J]. Environmental Research Letters, 2014, 9: 091001. DOI:10.1088/1748-9326/9/9/091001 |
| [7] |
Steinmetz Z, Wollmann C, Schaefer M, et al. Plastic mulching in agri culture: Trading short-term agronomic benefits for long-term soil degradation?[J]. Science of the Total Environment, 2016, 550: 690-705. DOI:10.1016/j.scitotenv.2016.01.153 |
| [8] |
韩作黎. 新华词典[M]. 北京: 商务印书馆, 2013. HAN Zuo-li. Xinhua dictionary[M]. Beijing: The Commercial Press, 2013. |
| [9] |
Gopferich A. Mechanisms of polymer degradation and erosion[J]. Biomaterials, 1996, 17(2): 103-114. |
| [10] |
Bonhomme S, Cuer A, Delort A, et al. Environmental biodegradation of polyethylene[J]. Polymer Degradation and Stability, 2003, 81: 441-452. DOI:10.1016/S0141-3910(03)00129-0 |
| [11] |
Shah A.A, Hasan F, Hameed A, et al. Biological degradation of plastics: A comprehensive review[J]. Biotechnology Advances, 2008, 26(3): 246-265. DOI:10.1016/j.biotechadv.2007.12.005 |
| [12] |
Ammala A, Bateman S, Dean K, et al. An overview of degradable and biodegradable polyolefins[J]. Progress in Polymer Science, 2011, 36(8): 1015-1049. DOI:10.1016/j.progpolymsci.2010.12.002 |
| [13] |
Briassoulis D, Babou E, Hiskakis M, et al. Degradation in soil behavior of artificially aged polyethylene films with pro-oxidants[J]. Journal of Applied Polymer Science, 2015, 132(30): 42289. |
| [14] |
Wilkes R A, Aristilde L. Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.:Capabilities and challenges[J]. Journal of Applied Microbiology, 2017, 123(3): 582-593. DOI:10.1111/jam.2017.123.issue-3 |
| [15] |
夏勇, 黄昌猛, 吕海霞, 等. 聚乙烯的结构、性能与应用[J]. 橡塑技术与装备(塑料), 2017, 43: 42-45. XIA Yong, HUANG Chang-meng, LÜ Hai-xia, et al. Structure, properties and applications of polyethylene[J]. China Rubber/Plastics Technology and Equipment (Plastics), 2017, 43: 42-45. |
| [16] |
潘祖仁. 高分子化学[M]. 北京: 化学工业出版社, 2015. PAN Zu-ren. Polymer chemistry[M]. Beijing: Chemical Industry Press, 2015. |
| [17] |
欧阳平凯, 姜岷, 李振江, 等. 生物基高分子材料[M]. 北京: 化学工业出版社, 2012. OUYANG Ping-kai, JIANG Min, LI Zhen-jiang, et al. Biobased polymeric materials[M]. Beijing: Chemical Industry Press, 2012. |
| [18] |
Gewert B, Plassmann M M, Macleod M. Pathways for degradation of plastic polymers floating in the marine environment[J]. Environmental Science: Processes & Impacts, 2015, 17(9): 1513-1521. |
| [19] |
Krueger M C, Harms H, Schlosser D. Prospects for microbiological solutions to environmental pollution with plastics[J]. Applied Microbiology and Biotechnology, 2015, 99(21): 8857-8874. DOI:10.1007/s00253-015-6879-4 |
| [20] |
Laycock B, Nikolić M, Colwell J M, et al. Lifetime prediction of biodegradable polymers[J]. Progress in Polymer Science, 2017, 71: 144-189. DOI:10.1016/j.progpolymsci.2017.02.004 |
| [21] |
陈松哲, 于九皋. LDPE/有机金属降解剂/配合剂体系降解性的研究[J]. 高分子材料科学与工程, 2001, 17(5): 140-143. CHEN Song-zhe, YU Jiu-gao. A study on a novel LDPE degradable system under simulating compost condition[J]. Polymer Materials Science and Engineering, 2001, 17(5): 140-143. DOI:10.3321/j.issn:1000-7555.2001.05.035 |
| [22] |
Albertsson A C, Karlsson S. The three stages in degradation of polymers-polyethylene as a model substance[J]. Journal of Applied Polymer Science, 1988, 35(5): 1289-1302. DOI:10.1002/app.1988.070350515 |
| [23] |
Ohtake Y, Ashabe H, Murakami N, et al. Biodegradation of lowdensity polyethylene, polystyrene, polyvinyl-chloride, and urea-formaldehyde resin buried under soil for over 32 years[J]. Journal of Applied Polymer Science, 1995, 56: 1789-1796. DOI:10.1002/app.1995.070561309 |
| [24] |
Ohtake Y, Kobayashi T, Asabe H, et al. Studies on biodegradation of LDPE: Observation of LDPE films scattered in agricultural fields or in garden soil[J]. Polymer Degradation and Stability, 1998, 60(1): 79-84. DOI:10.1016/S0141-3910(97)00032-3 |
| [25] |
Ohtake Y, Kobayashi T, Asabe H, et al. Oxidative degradation and molecular weight change of LDPE buried under bioactive soil for 32~ 37 years[J]. Journal of Applied Polymer Science, 1996, 70: 1643-1659. |
| [26] |
于红军, 赵英. 农用塑料制品与加工[M]. 北京: 科学技术文献出版社, 2002. YU Hong-jun, ZHAO Ying. Agricultural plastic products and processing[M]. Beijing: Scientific and Technical Documents Publishing House, 2002. |
| [27] |
代军, 晏华, 郭骏骏, 等. 结晶度对聚乙烯热氧老化特性的影响[J]. 材料研究学报, 2017, 31(1): 41-48. DAI Jun, YAN Hua, GUO Jun-jun, et al. Effects of crystallinity on degradation properties of polyethylene by thermo-oxidation aging[J]. Chinese Journal of Materials Research, 2017, 31(1): 41-48. |
| [28] |
Vasile C. Handbook of polyolefins[M]. Second edition. New York: CRC Press, 2000.
|
| [29] |
Balasubramanian V, Natarajan K, Hemambika B, et al. High-density polyethylene(HDPE)-degrading potential bacteria from marine ecosystem of Gulf of Mannar, India[J]. Letter in Applied Microbiology, 2010, 51: 205-211. |
| [30] |
Fontanella S, Bonhomme S, Koutny M, et al. Comparison of the biodegradability of various polyethylene films containing pro-oxidant additives[J]. Polymer Degradation and Stability, 2010, 95(6): 1011-1021. DOI:10.1016/j.polymdegradstab.2010.03.009 |
| [31] |
代军, 晏华, 郭骏骏, 等. 密度对聚乙烯光氧老化特性的影响研究[J]. 高分子通报, 2017(2): 46-55. DAI Jun, YAN Hua, GUO Jun-jun, et al. Influence of density on degradation properties of polyethylene after photo-oxidation aging[J]. Polymer Bulletin, 2017(2): 46-55. |
| [32] |
Roy P K, Hakkarainen M, Varma I K, et al. Degradable polyethylene: Fantasy or reality[J]. Environmental Science & Technology, 2011, 45(10): 4217-4227. |
| [33] |
Jakubowicz I. Evaluation of degradability of biodegradable polyethylene (PE)[J]. Polymer Degradation and Stability, 2003, 80(1): 39-43. DOI:10.1016/S0141-3910(02)00380-4 |
| [34] |
Chiellini E, Corti A, D′antone S, et al. Oxo-biodegradable carbon backbone polymers: Oxidative degradation of polyethylene under accelerated test conditions[J]. Polymer Degradation and Stability, 2006, 91(11): 2739-2747. DOI:10.1016/j.polymdegradstab.2006.03.022 |
| [35] |
Albertsson A C, Hakkarainen M. Designed to degrade[J]. Science, 2017, 358(6365): 872-873. DOI:10.1126/science.aap8115 |
| [36] |
Hayes D G, Wadsworth L C, Sintim H Y, et al. Effect of diverse weathering conditions on the physicochemical properties of biodegradable plastic mulches[J]. Polymer Testing, 2017, 62: 454-467. DOI:10.1016/j.polymertesting.2017.07.027 |
| [37] |
Albertsson A C, Erlandsson B, Hakkarainen M, et al. Molecular weight changes and polymeric matrix changes correlated with the formation of degradation products in biodegraded polyethylene[J]. Journal of Environmental Polymer Degradation, 1998, 6(4): 187-195. DOI:10.1023/A:1021873631162 |
| [38] |
Reddy M M, Deighton M, Gupta R K, et al. Biodegradation of oxo-biodegradable polyethylene[J]. Journal of Applied Polymer Science, 2009, 111(3): 1426-1432. |
| [39] |
Roy P K, Titus S, Surekha P, et al. Degradation of abiotically aged LDPE films containing pro-oxidant by bacterial consortium[J]. Polymer Degradation and Stability, 2008, 93(10): 1917-1922. DOI:10.1016/j.polymdegradstab.2008.07.016 |
| [40] |
Hadad D, Geresh S, Sivan A. Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis[J]. Journal of Applied Microbiology, 2005, 98(5): 1093-1100. DOI:10.1111/jam.2005.98.issue-5 |
| [41] |
Yang J, Yang Y, Wu W M, et al. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms[J]. Environmental Science & Technology, 2014, 48(23): 13776-13784. |
| [42] |
Restrepo-Flórez J-M, Bassi A, Thompson M R. Microbial degradation and deterioration of polyethylene: A review[J]. International Biodeterioration and Biodegradation, 2014, 88: 83-90. DOI:10.1016/j.ibiod.2013.12.014 |
| [43] |
Nowak B, Pająk J, Drozd-Bratkowicz M, et al. Microorganisms participating in the biodegradation of modified polyethylene films in different soils under laboratory conditions[J]. International Biodeterioration and Biodegradation, 2011, 65(6): 757-767. DOI:10.1016/j.ibiod.2011.04.007 |
| [44] |
Esmaeili A, Pourbabaee A A, Alikhani H A, et al. Biodegradation of low-density polyethylene (LDPE) by mixed culture of Lysinibacillus xylanilytics and Aspergillus niger in soil[J]. PLoS ONE, 2013, 8(9): e71720. DOI:10.1371/journal.pone.0071720 |
| [45] |
Watanabe T, Ohtake Y, Asabe H, et al. Biodegradability and degrading microbes of low-density polyethylene[J]. Journal of Applied Polymer Science, 2009, 111: 551-559. DOI:10.1002/app.v111:1 |
| [46] |
Sudhakar M, Doble M, Murthy P S, et al. Marine microbe-mediated biodegradation of low-and high-density polyethylenes[J]. International Biodeterioration and Biodegradation, 2008, 61(3): 203-213. DOI:10.1016/j.ibiod.2007.07.011 |
| [47] |
Seneviratne G, Tennakoon N S, Weerasekara M L, et al. Polyethylene biodegradation by a developed Penicillium-Bacillus biofilm[J]. Current Science, 2006, 90(1): 20-21. |
| [48] |
Kawai F, Watanabe M, Shibata M, et al. Comparative study on biodegradability of polyethylene wax by bacteria and fungi[J]. Polymer Degradation and Stability, 2004, 86(1): 105-114. DOI:10.1016/j.polymdegradstab.2004.03.015 |
| [49] |
Koutny M, Amato P, Muchova M, et al. Soil bacterial strains able to grow on the surface of oxidized polyethylene film containing prooxidant additives[J]. International Biodeterioration & Biodegradation, 2009, 63(3): 354-357. |
| [50] |
Rajandas H, Parimannan S, Sathasivam K, et al. A novel FTIR-ATR spectroscopy based technique for the estimation of lowdensity polyethylene biodegradation[J]. Polymer Testing, 2012, 31(8): 1094-1099. DOI:10.1016/j.polymertesting.2012.07.015 |
| [51] |
Koutny M, Sancelme M, Dabin C, et al. Acquired biodegradability of polyethylenes containing pro-oxidant additives[J]. Polymer Degradation and Stability, 2006, 91: 1495-1503. DOI:10.1016/j.polymdegradstab.2005.10.007 |
| [52] |
Gilan(Orr) I, Hadar Y, Sivan A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber[J]. Applied Microbiology and Biotechnology, 2004, 65(1): 97-104. |
| [53] |
Santo M, Weitsman R, Sivan A. The role of the copper-binding enzyme laccase in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber[J]. International Biodeterioration and Biodegradation, 2013, 84: 204-210. DOI:10.1016/j.ibiod.2012.03.001 |
| [54] |
Chatterjee S, Roy B, Roy D, et al. Enzyme-mediated biodegradation of heat treated commercial polyethylene by Staphylococcal species[J]. Polymer Degradation and Stability, 2010, 95(2): 195-200. DOI:10.1016/j.polymdegradstab.2009.11.025 |
| [55] |
Pometto A L, Lee B T, Johnson K E. Production of an extracellular polyethylene-degrading enzyme(s) by Streptomyces species[J]. Applied and Environmental Microbiology, 1992, 58(2): 731-733. |
| [56] |
Karlsson S, Ljungquist O, Albertsson A. Biodegradation of polyethylene and the influence of surfactants[J]. Polymer Degradation and Stability, 1988, 21: 237-250. DOI:10.1016/0141-3910(88)90030-4 |
| [57] |
Volke-Sepúlveda T, Saucedo-Castañeda G, Gutiérrez-Rojas M, et al. Thermally treated low density polyethylene biodegradation by Penicillium pinophilum and Aspergillus niger[J]. Journal of Applied Polymer Science, 2002, 83: 305-314. DOI:10.1002/app.v83:2 |
| [58] |
Pramila R, Vijaya R K. Biodegradation of low density polyethylene (LDPE)by fungi isolated from municipal landfill area[J]. Journal of Microbiology and Biotechnology Research, 2011, 1(4): 131-136. |
| [59] |
Pramila R. Biodegradation of low density polyethylene (LDPE) by fungi isolated from marine water: A SEM analysis[J]. African Journal of Microbiology Research, 2011, 5(28): 5013-5018. |
| [60] |
Sowmya H V, Ramalingappa M, Krishnappa M. Degradation of polyethylene by Chaetomium sp. and Aspergillus flavus[J]. International Journal of Recent Science Research, 2012, 3: 513-517. |
| [61] |
Albertsson A, Karlsson S. The influence of biotic and abiotic environments on the degradation of polyethylene[J]. Progress in Polymer Science, 1990, 15(2): 177-192. DOI:10.1016/0079-6700(90)90027-X |
| [62] |
Manzur A, Limon-Gonzalez M, Favela-Torres E. Biodegradation of physicochemically treated LDPE by a consortium of filamentous fungi[J]. Journal of Applied Polymer Science, 2004, 92: 265-271. DOI:10.1002/app.13644 |
| [63] |
Yamada-Onodera K, Mukumoto H, Katsuyaya Y, et al. Degradation of polyethylene by a fungus, Penicillium simplicissimum YK[J]. Polymer Degradation and Stability, 2001, 72(2): 323-327. DOI:10.1016/S0141-3910(01)00027-1 |
| [64] |
Awasthi S, Srivastava N, Singh T, et al. Biodegradation of thermally treated low density polyethylene by fungus Rhizopus oryzae NS 5[J]. 3 Biotech, 2017, 7: 73. |
| [65] |
Lucas N, Bienaime C, Belloy C, et al. Polymer biodegradation: Mechanisms and estimation techniques: A review[J]. Chemosphere, 2008, 73(4): 429-442. DOI:10.1016/j.chemosphere.2008.06.064 |
| [66] |
Wei R, Zimmermann W. Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: How far are we?[J]. Microbial Biotechnology, 2017, 10(6): 1308-1322. DOI:10.1111/1751-7915.12710 |
| [67] |
郭骏骏, 晏华, 包河彬. 聚烯烃老化评价实验方法评述[J]. 中国塑料, 2014, 28(4): 14-22. GUO Jun-jun, YAN Hua, BAO He-bin. An overview of evaluation testing methods on degradation of polyolefins[J]. China Plastics, 2014, 28(4): 14-22. |
| [68] |
陈厚, 郭磊, 李桂英, 等. 高分子材料分析测试与研究方法[M]. 北京: 化学工业出版社, 2011. CHEN Hou, GOU Lei, LI Gui-ying, et al. Investigation and analysis technology of polymer materials[M]. Beijing: Chemical Industry Press, 2011. |
| [69] |
Rajandas H, Parimannan S, Sathasivam K, et al. A novel FTIR-ATR spectroscopy based technique for the estimation of low density polyethylene biodegradation[J]. Polymer Testing, 2012, 31(8): 1094-1099. DOI:10.1016/j.polymertesting.2012.07.015 |
 2019, Vol. 38
2019, Vol. 38