农业生产中因氮肥不合理施用向大气中排放的活性氮气体主要包括NH3、N2O和氮氧化物(NO、NO2)。NH3作为碱性气体,能与大气中的NO2或SO2等酸性气体以及挥发性有机物发生反应而生成铵盐气溶胶,是空气雾霾形成的主要途径,对空气质量及人体健康造成负面影响[1];此外,挥发到大气中的NH3还能以干沉降形式再回到地面,对自然生态系统产生影响[2]。好气条件下土壤中氮素的硝化过程会产生N2O和NO,嫌气条件下土壤中氮素的反硝化过程也会产生N2O及氮氧化物(NO、NO2)。其中,N2O的温室效应是等质量CO2气体的265倍[3],是主要的农业源温室气体之一;土壤中产生的NO气体,进入到大气中会被氧气或臭氧等氧化剂氧化为NO2,进而与水反应形成硝酸型酸雨降落到地面[4]。化肥对全球及中国粮食增产的贡献分别为50%~60%和21%[5-6],我国农业生产中氮肥用量占到化肥总用量的40%,其中速效性氮肥占95%以上[7]。由于农民盲目追求产量及认知不足导致的不合理施肥所产生的活性氮排放给环境造成了各种负面影响,在一些经济发达地区尤为严重[8]。因此,提高氮肥肥效,降低活性氮对气候及环境的负面影响在全球范围内被广泛关注[4],目前,较为有效及成本适中的首选方法是合理控制氮肥用量及采用氮肥增效剂[9]。
氮肥增效剂主要包括脲酶抑制剂(UI)和硝化抑制剂(NI)两类,都与氮肥配合施用,以减少氮素损失、提高氮肥肥效。土壤中的铵态氮能被黏土矿物晶格所固定,也能被带负电的土壤胶体吸附而暂时保存起来;而硝态氮由于带负电,无法被带负电的土壤胶体吸附,很容易随降水或灌溉发生淋失而使氮素利用率降低[10-11]。UI主要配合尿素施用,抑制尿素水解为铵的过程,使尿素尽可能以酰铵态氮保持于土壤中,减少氨挥发和其后一系列氮素转化引起的可能损失及对气候和环境的负面影响[12]。NI是一类抑制氮素硝化作用过程的物质,硝化过程中铵态氮经羟胺及亚硝态氮转化为硝态氮,期间会排放N2O和NO,NI能不同程度地延长铵态氮在土壤中的存留时间,避免硝化作用向大气中排放N2O和NO,同时也减少随后因硝态氮淋洗或径流对地下水和陆面水体造成污染,及因反硝化作用继续排放N2O和其他氮氧化物,直接或间接降低氮肥施用对气候和环境的多方面风险[11, 13]。抑制剂用量一般只占到氮肥用量的0.5%~5%(由于施用方法及土壤、作物类型等不同,也有少数研究报道NI用量为氮肥用量的10%效果较好),价格比一般的缓释肥有优势,是一项有发展潜力的氮肥增效剂[9]。
本文对抑制剂的类型及新型抑制剂的发展、抑制剂作用机理及其对相关氮转化微生物的影响、抑制剂控制活性氮气体排放的效果及其影响因素进行综述,为提高氮肥利用率及降低氮肥施用中活性氮气体排放对气候及环境的负面影响提供科学参考。
1 肥料氮素在土壤中转化的主要过程及相关的微生物酶速效性氮肥主要包括酰胺态氮肥(如尿素)、铵态氮肥(如硫铵)、硝态氮肥(硝酸钙)、氨水、碳酸氢铵等,尿素是目前全球使用最为普遍的氮肥类型,特别是在发展中国家[7]。土壤中释放活性氮气体的过程主要包括尿素水解、硝化作用、反硝化作用等过程。
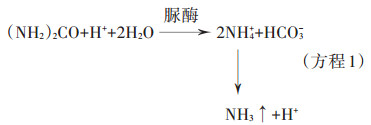
1.1 尿素水解过程与脲酶的作用尿素施入土壤后,很快在脲酶的作用下水解为铵态氮。脲酶也称酰胺水解酶,最适pH为7.4,能够专一性地催化尿素水解生成NH3和碳酸氢根,并短暂性地提高土壤环境的pH[14](具体反应见方程1)。脲酶结晶首次是从刀豆粉中分离纯化得到的,Sumner[15]因此获得了1946年诺贝尔化学奖。脲酶是一种含有镍离子的酶[16],在各种动植物、细菌和真菌中,特别是豆科植物中都广泛存在,是一种金属肽酶[17-18]。脲酶的分子量为480 000,有129个半胱氨酰基、47个巯基(半胱氨酰基残基),其中有4~8个巯基对脲酶活性起重要作用[17]。细菌的尿素酶是由2~3个不同亚基组成的杂聚肽,它包含γ、β和α 3个亚单位,分别由ureA、ureB和ureC基因编码,组成尿素原酶,但还不具有活化活性;需要一些辅助基因(包括ureD、ureE、ureF、ureG、ureH、ureI等)将镍离子转运到酶原才具有活性,活化后的脲酶含有3个催化部位,每个部位都含有2个镍离子。脲酶被激活后,各种辅助因子从酶上解离下来持续进入下一个循环[19-20]。
|
自养硝化作用是农业土壤中主要的硝化作用过程,该过程在硝化微生物的主导下完成,其中的第一步由具有氨单加氧酶(AMO)的氨氧化微生物参与,利用空气中的氧气将NH3先氧化为羟胺;之后由羟胺氧化还原酶(HAO)将羟胺继续氧化为亚硝酸根;接着,在亚硝酸盐氧化还原酶(NXR)的作用下继续将亚硝酸根氧化为硝酸根,硝化作用会向土壤中释放氢离子使土壤pH短暂下降[21-22](图 1)。其中,参与硝化作用第一步的氨氧化微生物包括氨氧化细菌(AOB)和氨氧化古菌(AOA)两大类,分别为碱性土壤和酸性土壤中的主要氨氧化微生物[23-24],该步骤是硝化作用的限速步骤。
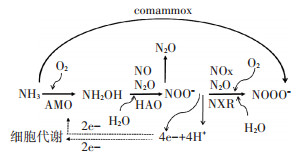
|
图 1 氮素硝化作用示意图 Figure 1 Diagram of nitrogen nitrification |
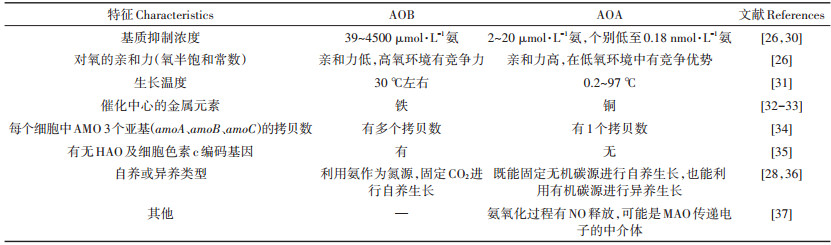
AOA和AOB的氨基酸序列相似度仅40%[25],二者在细胞结构及代谢途径方面还存在诸多差异(表 1)。研究表明,AOA为酸性土壤中的主要氨氧化微生物,有的AOA菌株能在pH为4的环境下进行氨的氧化[26]。AOA适应低pH环境,可能与其基因组中含有大量脲酶编码基因有关,使其能够利用环境中的脲素作为氨氧化的基质[26];此外,AOA可能利用低pH下质子化的氨做基质,也是它在酸性环境中优势明显的原因之一[27]。农业土壤中氨氮浓度在人为施肥后都较高,因此即使是AOA的数量级高于AOB,由于AOB的基质抑制浓度较高,其氨氧化反应的主导微生物仍是AOB。正是由于AOA和AOB的这些不同特征,各种UI及NI对硝化微生物和反硝化微生物的影响也不尽相同。AOB会由于环境中氨的缺乏而无法生长,pH每降低一个单位,非质子化的氨浓度会减少一个数量级,因此AOB在酸性环境中因基质氨的缺乏而不能正常生长[23]。由于AOA与AOB的上述不同特征,使AOA和AOB存在明显的生态位分异,AOA主要在低氮及pH较低的自然生态系统中占主导作用[27]。
|
|
表 1 AOB与AOA在细胞结构及代谢途径上的关键特征 Table 1 The key characteristics of AOB and AOA in the cell structure and metabolic pathways |
AMO除了能够氧化氨以外,还可以氧化C-H键和C=C键。编码AMO的基因簇位于一个操纵子中,至少含有3个基因(amoA、amoB和amoC),一个细胞中一般含3个拷贝的AMO,且不同拷贝之间有很高的保守性[28-31]。HAO是一种具有相同亚基的三聚体蛋白复合体,能够氧化羟胺,长度为1710 bp,受氨基和亚硝酸的诱导表达[31]。NXR是一种含有铁、钼、硫和铜离子的可溶性酶,基因簇中含有3个基因(nxrA、nxrB和nxrX),是由3个不同亚基组成的三聚体膜结合蛋白,在染色体上相邻排列并组成一个操纵子,3个基因的序列长度分别为3000、1539 bp和648 bp;NXR的催化作用是可逆的,受游离氨、亚硝酸及硝酸浓度、供氧状况等的影响[32-33]。
此外,科学家们于2015年发现了一种完全氨氧化细菌(Comammox Nitrosospira),能够直接将氨氧化为硝酸根,而且这种完全氨氧化菌对氨具有较高的亲和力,在贫营养环境中有竞争优势[34-36]。这类完全氨氧化菌的AMO在进化上与AOB和AOA的AMO不同,传统的16S rRNA或nxrB基因系统发育树分析法无法区分它与传统的硝化螺菌(Nitrospira),其AMO基因序列是穿插在严格的亚硝酸盐氧化的硝化螺菌属序列中,土壤中的完全氨氧化细菌基本属于Nitrospira谱系Ⅱ,对于亚硝酸盐的亲和力差[37-38]。在纯培养条件下,完全氨氧化细菌在富集培养时能够利用氨产生N2O,但土壤中该途径的发生条件及能否产生N2O还仍然未知[39]。
1.3 反硝化作用及其相关微生物反硝化作用是硝态氮在微生物作用下脱氮的过程,完全反硝化作用过程有4个步骤(图 2),参与各步骤的微生物酶分别为硝酸还原酶(Nar)、亚硝酸还原酶(Nir)、NO还原酶(Nor)及N2O还原酶(Nos)[40],其中关键的基因有nirS、nirK、nosZ。有的微生物具有完全反硝化作用的完整4类酶,而有的微生物则只分泌其中部分步骤的酶,特别是反硝化作用中最后一个步骤的酶Nos,其丰度高低及类型(nosZ Ⅰ型或nosZ Ⅱ型)对反硝化作用过程的最终产物比(N2O/N2)起重要作用。研究发现,NI对反硝化微生物功能基因的丰度也有一定影响[41]。
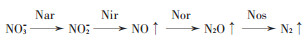
|
图 2 氮素反硝化作用示意图 Figure 2 Diagram of nitrogen denitrification |
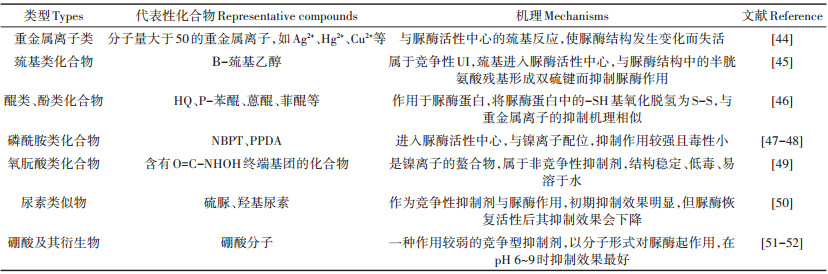
19世纪40年代,Conrad发现某些物质可以抑制土壤脲酶活性并延长尿素的有效期。60年代开始,发现含硼化合物、原子量大于50的重金属、含氟化合物、多元酚、多元醌和抗代谢物质类等对脲酶活性均有抑制作用,但由于各种UI作用的发挥受到诸多条件的限制,使得不能投入实际生产应用中。70年代,研究者们对百余种化合物进行了测试,Bremner[12]根据早期的研究筛选出抑制效果较好的苯醌类和氢醌类化合物,其中氢醌(HQ)因其较高的性价比在国际上得到了广泛研究和应用。进入80年代,国外开发了醌类、多羟酚类、磷酰胺类、五氯硝基苯等具有实用意义的UI。1996年,美国IMC-Agrico公司推出了活性成分为N-丁基硫代磷酰三胺(NBPT)的Agrotain系列添加剂,可用于固体尿素和硝铵尿素液体肥料,其溶剂为含10%的N-甲基吡咯烷酮及无毒害的惰性缓冲溶液[42]。由于重金属离子易造成环境污染,使其利用受到了一定限制,目前常用的UI为有机化合物类,主要为HQ、NBPT、苯基磷酰二胺(PPDA)、硫脲等。
从作用机理来看,UI可大致分为3大类,一类是尿素类似物(如硫脲、羟基尿素),作为尿素的竞争性抑制剂与脲酶作用;第二类是能与脲酶活性中心的巯基反应,使脲酶结构发生变化而失活的物质,如巯基类化合物、醌类、酚类化合物等;第三类化合物能与脲酶活性中心的镍离子配位,使得脲酶失活。脲酶分子活性中心有2个镍离子,其中一个镍离子在结构上是五配位的准四方锥构型,另一个镍离子是六配位的准八面体构型[43]。不同的抑制剂可能作用于脲酶活性中心的镍离子,也可能作用于其中的巯基,不同的结合方式使得活性中心形成空间位阻,从而抑制脲酶对尿素进行作用。表 2列出了主要的UI及其作用机理。
|
|
表 2 常见的脲酶抑制剂 Table 2 The commonly used urease inhibitors |
人工合成的NI按照化学形态分为无机化合物和有机化合物两大类。无机NI以重金属化合物为主,有机NI主要分为含硫化合物、乙炔及乙炔基取代物、氰胺类化合物、杂环氮化合物。常见的NI见表 3,生产中常用的NI有以下几种:氯啶类化合物,如2-氯-6-三氯甲基吡啶(Nitrapyrin),1974年被美国DOW公司开发为产品,商品名为N-server,在美国玉米种植带应用较广[53];双氰胺(DCD),由前西德SKW公司发展为商品名为Didin的产品[54];3,4-二甲基吡唑磷酸盐(DMPP),于1999年由德国BASF公司开发为产品,商品名为Entec[55]。
|
|
表 3 硝化抑制剂的类型及抑制机理[56] Table 3 The types of nitrification inhibitors and their mechanisms for inhibiting nitrification[56] |
土壤中硝化作用由微生物主导,NI通过直接影响硝化微生物的酶活性从而抑制土壤中氨氧化或亚硝化过程,其抑制途径主要有以下几种[22, 57]:
(1)将amoA中心的辅助因子铜螯合,从而抑制氨氧化微生物的活性[58-59],如硫脲。
(2)属于底物氨的竞争性抑制剂,能够与底物氨结合,从而阻止微生物与氨结合,其中一类是竞争性抑制剂,即其结合氨的位点与AMO结合氨的位点相同,也就是氨的类似物[60],这类NI有DCD、DMPP等;另外一类物质与氨的结合是非竞争性的,其结合位点与AMO结合氨的位点不同,该类物质是一类非极性的疏水物质,通过与其结合增大了AMO的原子间排列空间位阻而阻止AMO与氨的结合[22, 60-61],如烷烃类物质。
(3)一类基于机制的抑制剂,其与AMO蛋白不可逆结合,通过共价修饰AMO蛋白使AMO失活,这些失活的蛋白需微生物重新合成才能再产生。如乙炔能使AMO中的一个单一蛋白被结合而永久失活[62-63];而氯仿则能使AMO的多种细胞组成成分被共价结合而抑制AMO的活性[22],因此较之单一蛋白被结合而使AMO失活更难被恢复[64]。
大部分的NI通过抑制硝化作用过程中第一个步骤(氨氧化过程)中微生物的AMO酶活性而达到抑制硝化作用的效果,而对NXR步骤则没有抑制效果。不过,一些生物硝化抑制剂(BNI)能同时抑制氨氧化过程和羟胺氧化过程,其作用强度和持续时间都优于人工合成NI,如植物合成的野樱素、亚油酸、带苯环的脂肪酸化合物都能同时抑制AMO和HAO,且有的抑制作用可长达数月[65]。
2.3 生物硝化抑制剂BNI是自然界中某些植物根系分泌的具有较强硝化抑制能力的物质,Munrro于1966年在低氮的森林和草地土壤中发现,一直到2006由Subbarao创立了以相当于0.22 μmol·L-1的化学抑制剂烯丙基硫脲(AT)为单位的生物发光法才开启了其量化研究[66-67]。一般的NI都只能抑制硝化作用第一步反应的微生物酶AMO,而高粱及湿生牧草分泌的BNI能同时抑制AMO及HAO的活性[68]。
非洲臂形牧草、高粱、小麦、水稻、豆类作物等植株均可分泌BNI[67-69],这些抑制剂的类型有不饱和脂肪酸、野樱素、癸二醇等。高粱根系分泌物中的对羟基苯丙酸甲酯(MHPP),是一种具有BNI活性的苯丙酯类化合物[70],可能是好气的低氮生态系统中植物的一种自我适应能力。在高粱根系分泌物中还发现两种具有BNI活性的化合物Sorgoleone(一种苯醌类化合物),是疏水组分中主要的BNI;而Sakuranetin(一种黄烷酮类化合物)则是从高粱根系分泌物的亲水组分中分离出来的[71]。此外,还有来自信号草Brachiaria humidicola的根系分泌物Brachialactone(一种环二萜类化合物),由一个双环戊二烯、一个环辛烷骨架、一个桥接五元环和八元环的γ-内酯环组成[68, 72]。
影响植物分泌BNI的因素有氮素类型及浓度、土壤pH、硝化微生物类型及存在与否等。Zhang等[73]报道,低浓度的铵态氮(0.1 mmol·L-1)诱导下,水稻根系癸二醇的分泌量是对照的2.4倍,低pH以及改善通气状况都会促进水稻根系分泌1,9-癸二醇(例如癸二醇的分泌量在pH 3.0下是pH 7.0时的2倍);此外,改善通气状况使癸二醇的分泌量增加了63%。水稻分泌的这种癸二醇主要通过抑制AMO活性而抑制硝化作用,并且在水稻中发现了与非洲湿生臂形牧草相似的BNI编码基因[74]。
同样,BNI由于延长了氮素以铵态氮形式存在于土壤中的时间,也对降低因硝化和反硝化作用排放的N2O及减少硝态氮淋洗有显著作用。研究表明,BNI与添加Nitrapyrin的处理相比,N2O排放量无显著差异,其作用与化学NI相当[75]。还有试验表明,非洲臂形牧草根际的N2O比大豆根际N2O排放量低90%[68],其抑制硝化作用的能力更优于人工合成NI。
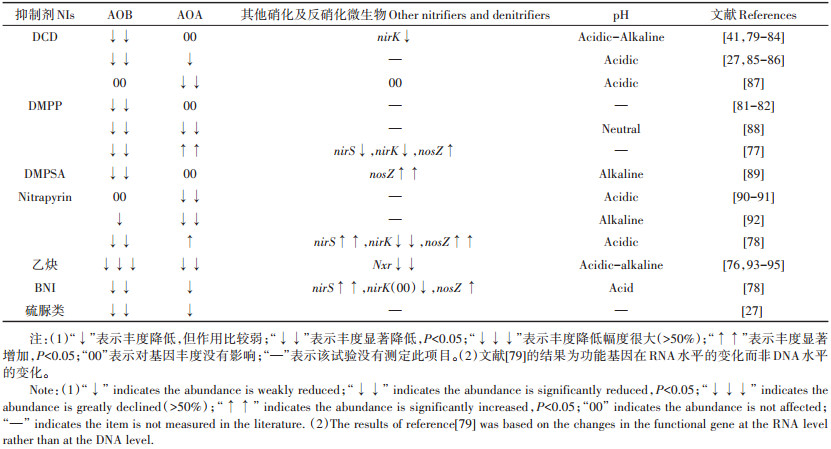
3 抑制剂对硝化微生物的影响由于不同抑制剂的作用机理不同(螯合酶活中心的金属离子、竞争性抑制剂、非竞争性抑制剂、对酶活中心的空间位阻等),硝化微生物对其的敏感性也不尽相同,因此,各种抑制剂作用的有效浓度及在不同的环境条件下的作用效果有一定差异。不同抑制剂对AOB和AOA的丰度、多样性及区系组成的影响,是随近些年高通量测序及宏基因组技术迅速发展而兴起的一个研究热点。
有研究指出,不同抑制剂对氨氧化微生物的抑制强弱大体为乙炔>DMPP>硫脲>Nitrapyrin,其中,乙炔不仅对AMO功能基因有抑制作用,而且能抑制NXR功能基因[76]。乙炔在生产中一般包被于碳化钙内使用,国内应用不多。由于一些NI本身也含有氮素,也是一种氮源或碳源,刚施入土壤中可短期被反硝化微生物作为电子供体而影响反硝化微生物的一些细胞代谢活动,对反硝化作用不同作用步骤的微生物有一定影响[77-78],而其对来自反硝化作用的N2O/N2的影响则取决于其对不同步骤及类别的反硝化微生物的相对影响程度,目前还没有足够的研究积累进行总结。
表 4总结了近年发表的关于抑制剂对硝化微生物影响的一些报道(关于UI,只收集到硫脲的报道),汇总结果显示,大部分NI都使中性至碱性土壤中AOB的功能基因丰度显著降低;而抑制剂对于土壤中AOA的影响,则两方面的报道都有,可能与土壤pH、基质浓度、施肥后取样测定微生物丰度的时期、土壤黏粒含量、土壤水分状况、土壤有机质含量等诸多因素有关。目前的研究报道数量还很有限,而且绝大部分的测定都是针对功能基因的DNA水平进行的,而非RNA水平的测定,因此,其反映的土壤中相关微生物的状况可能与其实际具有活性的微生物水平还不尽一致,还需要再积累更多不同条件下的研究数据及具有活性的微生物功能基因的检测研究,以进行进一步的分类汇总。
|
|
表 4 主要的硝化抑制剂对硝化微生物及部分反硝化微生物功能基因丰度的影响 Table 4 Effects of prevalent NIs on the abundance of functional genes of nitrifiers and some denitrifiers |
关于NI对完全氨氧化菌的影响,目前的研究还很少,据Li等[95]报道,对pH为近中性的澳大利亚农业菜地和草地2个土壤的研究显示,乙炔对2种土壤的完全氨氧化菌都有显著的抑制作用,其丰度降低了50%以上,尤其对农业土壤的完全氨氧化菌丰度抑制作用更强(丰度降低了近70%);而二环辛烷对完全氨氧化菌丰度的影响不显著。该研究还显示,乙炔使2种土壤的AOB和AOA丰度也都显著降低(10%到50%以上),其中AOB丰度降低的幅度比AOA更大,而且在农业土壤上的抑制效果高于草地土壤。
由于UI主要是抑制尿素的水解,一些硝化微生物也含有脲酶基因,因此一些UI对硝化微生物丰度也有一定影响,但这方面仅有几例报道[84, 91, 96],这里暂不赘述。
4 抑制剂对土壤活性氮气体排放的影响由于UI主要是抑制尿素水解为氨的过程,因此其抑制氨挥发的作用是直接的;而其抑制N2O和NO排放则是由于抑制尿素水解后的间接作用,即土壤中硝化作用的基质较低的缘故。由于硝化作用同时释放N2O和NO,因此能抑制硝化作用的抑制剂,理论上对NO也具有减排作用[97-99]。目前关于NI对N2O减排的报道较多,而对NO减排及3种活性氮气体同步监测的试验很少。下面就目前查阅的一些文献报道做简要汇总。
4.1 抑制剂对氨挥发的减排/促进效果UI对降低氨挥发有显著作用,通过对Ti等[100]文章中的数据集进行重新分类汇总,得出不同的UI对氨挥发减排的效果(与未施用UI相比)依次为:PPDA(-47%)>NBPT(-46%)>氨基硫脲(-17.7%)>HQ(-1.8%)。可见,抑制机理为螯合脲酶活性中心镍离子的抑制剂,其减少氨挥发的效果最显著,如磷酰胺类化合物PPDA及NBPT;而醌类化合物对氨的减排作用较弱。Pan等[101]的meta分析结果与此类似。
NI单独施用,一般对氨挥发有促进作用。我们对文献数据集[102]进行再汇总得到不同NI对氨挥发的促进作用为:N - server(194.2%,范围29%~359%)> DCD(44.8%,范围18.2%~71.5%)>DMPP(5.3%,范围-0.7%~11.3%),可见DMPP对氨挥发的促进作用最小。其他文献[101]汇总结果与此类似。
由于不同作物所处的气候区(气温、降水)、环境条件(土壤类型、土壤pH、黏粒含量等)及相应的管理措施(施肥方式、耕作措施、灌溉与否)不尽相同,抑制剂对种植不同作物农田的氨挥发减排或促进作用有所差异。Ti等[100]的meta分析结果显示,施用UI对不同土地利用类型的氨挥发降低的效果表现为稻田(63%)>小麦地(53%)>玉米地(44%)>草地(43%);而单独施用NI对不同土地利用类型的氨挥发总体都有增加趋势(有些研究结果也有减排氨的情况),具体为草地(101%,范围-10%~351%)>蔬菜地(49%,范围-46%~312%)>小麦地(31%,范围-43%~204%)>稻田(22%,范围-25%~105%)>玉米地(1%,范围-99%~49%)。
4.2 抑制剂对N2O及NO的减排效果从理论上来讲,能抑制氨挥发的UI及抑制硝化作用的NI,都能对NO有协同减排作用,因为NO与N2O都在硝化作用及随后的反硝化作用过程中产生。目前的研究中,关于抑制剂对NO减排的文献报道不多,已有的一些报道汇总如下:
(1)UI对N2O和NO的协同减排:Akiyama等[103]发现,UI(间二氮杂苯)使N2O和NO分别减排22.6%和36.1%。据Guardia等[99, 104]的报道,NBPT在2个不同年份使灌溉玉米地的N2O和NO分别降低了10.1%~57.6%和45.3%~71.3%,使雨养小麦地的N2O和NO排放分别降低了24.5%~63.3%和3.8%~10.9%。
(2)NI对N2O和NO的协同减排:Akiyama等[103]根据收集到的5组研究数据得出,NI(DCD和DMPP)使N2O和NO分别减排8.8%~46.7%和25.7%~73.8%。此外,田间试验表明,NI(Nitrapyrin和DMPSA)分别使雨养小麦地的N2O和NO分别减排12.9%~43.2%和49.2%~81.2%,且DMPSA的减排效果优于Nitrapyrin[104]。还有研究显示,DMPSA还能使施用硝酸铵钙的灌溉玉米地N2O和NO分别降低57.9%和12.5%~76.2%[99]。
(3)单一抑制剂对N2O减排的效果:生产上常用抑制剂的减排效果总体上表现为DMPP>NBPT>DCD≈ Nytrapirin。如徐玉秀等[105]的meta分析数据显示,几种抑制剂对N2O减排的效果依次为DMPP(51.1%)>NBPT(44.1%)>Nitrapyrin(39.5%)≈DCD(38.9%)。Ruser等[56]的meta分析显示,不同抑制剂的N2O减排效果依次为碳化钙(约46.5%)>DMPP(约37.5%)>Nitrapyrin(约32.5%)>硫脲(约22.0%)。Thapa等[106]的汇总分析结果为碳化钙(约52%)>DCD(43.5%)≈Nitrapyrin(约41%)>DMPP(33%)>硫脲(25%)>NBPT(20%)。
4.3 抑制剂配合施用对活性氮气体的减排效果由于UI对尿素水解和NI对硝化作用的抑制作用,UI和NI配合施用对N2O及NO减排的作用总体优于单一抑制剂施用的效果。例如,徐玉秀等[105]根据收集到的86组观测数据的汇总结果显示,不同抑制剂或抑制剂组合的N2O减排能力(与无抑制剂相比)依次为HQ+DCD(58.9%)>NBPT+DCD(52.9%)>其他UI及NI单一施用。
一些为数不多的同步监测氨挥发和N2O的研究显示,UI和NI配合施用对氨挥发和N2O总体减排的作用显著,同时对减少硝态氮淋洗也有显著作用。如Jiang等[107]的试验显示,UI与NI配合施用使长江中下游小麦地的NH3及N2O总减排量为51.4%,其对活性氮气体的综合减排作用相当于价格高昂的缓释肥的效果,此外还比单一抑制剂显著降低硝态氮淋洗,具有综合减少氮素多途径损失及总损失量的作用。孙祥鑫等[108]的研究表明,与单施尿素相比,水田土壤施用HQ、NBPT和DCD的氨挥发量分别减少了40.2%、43.2%和25.5%,N2O排放量分别降低了28.9%、17.7%和35.9%,而施用DMPP的氨挥发量增加了25.8%;施用HQ+DCD和NBPT+DMPP的氨挥发量分别减少了46.9%和71.9%,N2O排放量也分别减少了54.7%和42.2%,分别比各抑制剂单独施用对2种活性氮气体的减排效果都更好。
实际生产中一般会联合使用UI和NI,将其作为包膜材料包被于尿素颗粒外,谓之稳定性肥料,其对减少农田活性氮气体排放,同时显著减少硝态氮淋洗及提高作物产量的作用也比较显著[102, 109-110],是综合提高氮肥利用率且达到环境友好目标的合理选择。
5 影响抑制剂作用效果的因素抑制剂抑制农田活性氮气体排放的效果除取决于抑制剂的抑制机理,同时还受温度、土壤水分状况、土壤类型、土壤pH、土壤质地、土壤有机质含量等诸多因素的影响。下面就抑制剂对抑制尿素水解及硝化作用的主要影响因素及对活性氮气体减排的可能影响进行阐述,为生产中更好地发挥抑制剂的作用提供科学参考。
5.1 温度尿素水解是一种酶促反应,温度升高一方面加快了酶分子的热运动[111],降低酶活性中心与底物分子形成复合体时的能级障碍[112];另一方面,加快了UI自身的分解转化,减弱UI对脲酶的抑制作用,进而加快酶-底物复合体的形成和解离[113]。有研究表明,土壤温度升高,尿素的水解转化作用增强,UI的有效作用时间降低,抑制效应减弱[114],其他研究也都证明了这一点[55, 115-117]。
温度一般是通过影响NI的生物降解速率从而影响其抑制效果;但从另一方面来讲,温度低时硝化作用也弱,因此温度对抑制剂抑制效果的影响需综合分析。Menéndez等[118]的试验表明,抑制剂在高温下容易分解,因此其效果一般在较高温度时略低,且作用时间缩短。DCD对于温度较为敏感,而且DCD本身也是一种氮源,在土壤温度高于25 ℃时易被微生物降解,影响其作为抑制剂的效果[119]。还有试验表明,DMPP在高温下容易分解,影响其作用效果,3个不同的温度水平(15、25 ℃和35 ℃)下,15 ℃下DMPP的作用效果最佳,并可维持40 d左右[120]。
5.2 土壤水分土壤水分状况影响尿素水解的速率及尿素的保留时间,进而影响UI的抑制效果。有研究报道,NBPT的抑制作用随着时间的变化而降低,与土壤水分含量增加有关[121]。周旋等[114]研究显示,尿素水解时间表现为60% WHC(9 d)>80% WHC(6 d),脲酶抑制率也表现为60% WHC(45.9%)>80% WHC(19.5%),说明随土壤含水量的降低,尿素水解速度降低,使得尿素有效作用时间延长,脲酶抑制效应也增强。有研究报道,在7 mm和14 mm降水的土壤水分状况下,NBPT分别使氨挥发降低了77%和89%[122]。这说明,抑制剂的作用不仅与水分含量对抑制剂自身的抑制作用发挥有关,还与农田在不同水分含量下氨挥发的背景值有关。Sanz-Cobena等[123]的研究也表明,在60% WFPS下,NBPT和苯基磷二酰胺使N2O分别减排了83.2%和33.7%,NBPT抑制效果可长达9 d,苯基磷二酰胺只持续了3 d,而在80% WFPS下,两种UI都没有减少N2O的排放。
土壤水分对NI的抑制效果因NI本身特性的不同而存在差异。Nitrapyrin在较湿的土壤中,会向土壤下层移动,在较干的土壤中,则会向表层移动,且在湿润条件下,其挥发损失量也更大[124-125]。苯并三唑的硝化抑制效果不受土壤水分的影响,这与苯并三唑在土壤中不易被降解有很大关系[125]。而硝基酚、硝基苯胺、DCD易被土壤微生物利用,而且DCD的水溶性很高(13 ℃下水溶解度为22.6 g·L-1),在土体中移动性强,在遇强降雨情况下,容易发生与铵根分离的现象,甚至淋溶损失,从而影响其硝化抑制效果,还对环境造成影响[55]。研究发现,DCD在土壤含水量为12%和24%时的抑制作用时长分别为90 d和60 d[126]。
5.3 土壤pHNBPT是实际生产中应用较多的UI,有研究报道,NBPT在pH分别为5.1、6.1、7.6和8.2的未灭菌土壤中,半衰期分别为0.07、0.59、2.70 d和3.43 d[127]。Engel等[127-128]发现,NBPT的有效时长表现为碱性土(>7周)大于酸性土(2~3周),抑制率也表现为碱性土(86.2%)大于酸性土(17.0%),且与温度相关。还有研究表明,对于碱性土,施用NBPT显著降低了土壤铵态氮含量,并维持了25 d,最大抑制率高达86.5%,相应地,土壤硝态氮含量也降低,并维持了48 d;而在酸性土中,NBPT显著抑制土壤铵态氮产生的作用仅维持了1 d,最大抑制率也仅为44.3%[129]。
土壤pH对NI抑制效果的影响较为复杂,一般来讲,中性或石灰性土壤中的硝化作用比较强烈[130],NI的作用可能会比较显著;但在中性或微碱性土壤中,微生物的活性也较强,对NI的分解也有可能会增加[131]。pH降低会使环境中的铵发生质子化,降低基质的生物可利用性;pH也主导着土壤中AOB和AOA的生态位,不同微生物对其的敏感度不一样。Thapa等[106]的文献汇总结果显示,NI在不同土壤pH条件下降低N2O排放的效果依次为(括号中数字为添加抑制剂与不添加抑制剂的N2O相对减排比例):酸性土(57%)>中性土(44%)>碱性土(19%)。赖晶晶等[132]的研究结果显示,4种主要的NI均能抑制土壤铵态氮向硝态氮的转化和降低N2O的排放,但抑制效果存在差异,对碱性土的硝化速率抑制效果表现为MHPP> Nitrapyrin>DMPP>DCD,对酸性土则表现为MHPP> Nitrapyrin>DCD>DMPP。还有研究表明,在以AOB为主导的土壤中,Nitrapyrin和NBPT在pH>5时可以抑制AOB丰度,并随着pH升高(pH 3.97~7.04)抑制效果增强,其他研究针对Nitrapyrin、DCD及DMPP等的结果与此类似[82, 90]。
5.4 土壤质地土壤质地决定土壤的孔隙条件,从而影响土壤的通气性、水分含量以及有机质分解速率,进而影响土壤微生物的生长环境和基质供应。周旋等[133]的研究结果显示,NBPT和NPPT对不同质地土壤中的尿素水解及其抑制效应表现为砂土>黏土>壤土,而对表观硝化率及硝态氮含量抑制效应则表现为砂土>黏土≈壤土,这与其对活性氮气体减排效果直接相关,并且与不同质地土壤中产生的活性氮气体在土壤中的逃逸扩散能力或土壤孔隙度有一定关系[134-135]。
硝化微生物一般都生活在土壤团聚体内部,因此,黏粒含量较高的土壤由于土壤微域对硝化微生物存在一定保护作用,有可能避免抑制剂对硝化微生物活性的抑制作用[136]。DCD及DMPP在抑制氮转化方面的作用,在砂土上的抑制效果高于壤土和黏土[41, 137]。Thapa等[106]的文献汇总结果显示,NI对N2O减排的效果在不同质地土壤上依次为:砂土(48%)>中等质地土(40%)>黏土(38%)。
5.5 土壤有机质含量有机质含量高的土壤,其有机质对抑制剂有一定的吸附,该吸附一方面有利于抑制剂在土壤中的存留;但另一方面,又使得抑制剂受到了非生物学保护,且有机质含量高的土壤,反硝化微生物的活性也较强,抑制剂对土壤N2O排放的综合作用需全面考虑。有研究表明,NBPT对尿素水解的抑制效果往往会随着土壤有机质含量的减少而增加[115, 138]。Suter等[139]的研究显示,在土壤有机碳含量较高(11%)的草地土壤中,施用NBPT对尿素的水解速率影响不大,第14 d时尿素残留率小于1%;而在土壤有机碳含量较低(<1.5%)的小麦地中,施用NBPT能有效降低尿素水解,第14 d时尿素残留率大于55%。
Barth等[140]和Zhang等[141]的研究均表明,抑制剂被土壤有机质吸附,可有效防止其在短时间内被土壤微生物降解和挥发,起到延长抑制时长的作用。有研究发现,去除有机质后土壤DMPP半衰期是原土壤的3.8倍,显著延长了DMPP的存留时间,从而显著降低土壤表观硝化率[142]。另外,有的AOA可以利用有机物进行生长[143];而有的AOA则可利用无机碳进行生长,有机物反而会抑制其生长[26];还有一种情况是,有机物可以充当氨氧化微生物生长代谢过程中的一些代谢酶,类似促进微生物生长的催化剂的作用,所以对AOA可能起一定的促进作用[144]。因此,土壤中硝化微生物和反硝化微生物的群落组成结构,主要与土壤类型有关[145],抑制剂及各种有机物不会明显改变其微生物群落组成结构,但可能会对其组成比例有一定影响。
5.6 其他抑制剂的作用效果还与抑制剂的用量、施用方法、氮肥类型、农田管理措施、作物类型等有关。在一定范围内,随着UI用量的增加,其抑制效果逐渐增强[146]。张文学等[147]发现,随着NBPT添加量的增加,土壤脲酶活性递减,当添加量超过尿素的1%时,效果显著。然而,也有研究指出,有的土壤在PPD和NBPT的添加量达到尿素的10%时对尿素的水解才有显著的抑制效果[148]。UI与尿素配合深施能显著降低氨挥发,meta分析结果显示,UI与肥料深施和表施对氨挥发减排的效果分别为46%和37%[106, 149]。当抑制剂未与土壤充分混匀,而是与氮肥表施时,其抑制效果通常较差,且抑制剂的添加量需要增加,这与抑制剂的挥发、降解及移动速度有关,如Nitrapyrin易见光分解为6-氯-吡啶甲酸,造成挥发损失,不适合表施[150]。研究表明,田间条件下,DMPP施用量为0.5~1.5kg·hm-2时,其抑制时间可持续4~10周[151]。DMPP的抑制效果和有效时长随着DMPP用量的增加而增强,但当DMPP增加到2%以上时,其抑制效果不再明显[120]。也有研究显示,DCD和Nitrapyrin均能够显著降低石灰性土壤N2O的排放,但施用2倍推荐量的Nitrapyrin并没有显著降低N2O排放量[152]。
6 展望在多种措施综合利用(比如结合肥料深施、多次施用等管理措施[106])的情况下,人工合成的各种化学抑制剂对抑制氮素转化过程中向大气释放活性氮的降低作用可以达到与缓释肥类似的效果[100],而其成本比缓释肥低,推广应用方面容易实现。此外,研究适合于水肥一体化系统的液体NI、大幅控制果树及蔬菜等氮肥用量较高的生产系统中的活性氮气体排放等方面也应该得到关注。
由于大部分BNI既能抑制氨氧化过程,也能抑制羟胺氧化过程[70],而且还对侧根生长有促进作用[153],对环境的毒害较小,其未来应用前景可观。筛选适合不同作物生长系统(如旱地、稻田)的适宜BNI,研究其分子结构、抑制机理、适宜浓度、植物产生这些BNI的影响因素及对作物生长的其他相关作用,以及对氮素转化中N2O/NO排放比值、氨挥发及硝态氮淋洗方面的同步监测与评估也需要开展更多的研究工作。此外,借助分子生物学技术,将具有高BNI表达能力的植物基因转入无分泌能力或低分泌能力的作物中,通过分子生物学技术降低土壤硝化作用的强度,并对土壤活性氮气体排放起到抑制作用,将是提高氮素利用率、降低氮肥施用向大气排放活性氮气体的可能途径[154]。
此外,由于不同氨氧化菌(AOB、AOA、完全氨氧化菌)的生态位条件不同,研究各种抑制剂对这些硝化微生物功能基因丰度、多样性及群落组成结构的影响及其机理,以及这些影响与各种环境因子的关系,对进一步科学控制氮素硝化作用过程中活性氮的排放,也将会有更进一步的认识。
| [1] |
Ellis R A, Murphy J G, Markovic M Z, et al. The influence of gas-particle partitioning and surface-atmosphere exchange on ammonia during BAQS-Met[J]. Atmospheric Chemistry and Physics, 2011, 11(1): 133-145. |
| [2] |
Behera S N, Sharma M, Aneja V P, et al. Ammonia in the atmosphere:A review on emission sources, atmospheric chemistry and deposition on terrestrial bodies[J]. Environmental Science and Pollution Research International, 2013, 20(11): 8092-8131. DOI:10.1007/s11356-013-2051-9 |
| [3] |
IPCC. The fifth assessment report of the intergovernmental panel on climate change[R]. Cambridge: IPCC, 2013.
|
| [4] |
Galloway J N, Townsend A R, Erisman J W, et al. Transformation of the nitrogen cycle:Recent trends, questions, and potential solutions[J]. Science, 2008, 320(5878): 889-892. DOI:10.1126/science.1136674 |
| [5] |
奚振邦. 化肥与农业——简析化肥对现代农业的作用[J]. 磷肥与复肥, 2003, 18(2): 5-10. XI Zhen-bang. Fertilizer and agriculture:A brief review on the effect of fertilizer on modern agriculture[J]. Phosphate & Compound Fertilizer, 2003, 18(2): 5-10. DOI:10.3969/j.issn.1007-6220.2003.02.002 |
| [6] |
房丽萍, 孟军. 化肥施用对中国粮食产量的贡献率分析-基于主成分回归C-D生产函数模型的实证研究[J]. 中国农学通报, 2013, 29(17): 156-160. FANG Li-ping, MENG Jun. Application of chemical fertilizer on grain yield in China analysis of contribution rate:Based on principal component regression C-D production function model and its empirical study[J]. Chinese Agricultural Science Bulletin, 2013, 29(17): 156-160. DOI:10.11924/j.issn.1000-6850.2012-3807 |
| [7] |
张卫峰, 马林, 黄高强, 等. 中国氮肥发展、贡献和挑战[J]. 中国农业科学, 2013, 46(15): 3161-3171. ZHANG Wei-feng, MA Lin, HUANG Gao-qiang, et al. The development and contribution of nitrogenous fertilizer in China and challenges faced by the country[J]. Science Agriculture Sinica, 2013, 46(15): 3161-3171. DOI:10.3864/j.issn.0578-1752.2013.15.010 |
| [8] |
Liu X J, Xu W, Duan L, et al. Atmospheric nitrogen emission, deposition, and air quality impacts in China:An overview[J]. Current Pollution Reports, 2017, 3: 65-77. |
| [9] |
熊舞, 夏永秋, 周伟, 等. 菜地氮肥用量与N2O排放的关系及硝化抑制剂效果[J]. 土壤学报, 2013, 50(4): 743-751. XIONG Wu, XIA Yong-qiu, ZHOU Wei, et al. Relationship between nitrogen application rate and nitrous oxide emission and effect of nitrification inhibitor in vegetable farming system[J]. Acta Pedologica Sinica, 2013, 50(4): 743-751. |
| [10] |
巨晓棠, 刘学军, 张福锁. 尿素与DCD和有机物料配施条件下氮素的转化和去向[J]. 中国农业科学, 2002, 35(2): 181-186. JU Xiao-tang, LIU Xue-jun, ZHANG Fu-suo. Nitrogen transformation and fate in soil under the conditions of mixed application of urea with DCD or different organic materials[J]. Scientia Agricultura Sinica, 2002, 35(2): 181-186. DOI:10.3321/j.issn:0578-1752.2002.02.013 |
| [11] |
Wang D Y, Guo L P, Zheng L, et al. Effects of nitrogen fertilizer and water management practices on nitrogen leaching from a typical open field used for vegetable planting in northern China[J]. Agricultural Water Management, 2019, 213: 913-921. DOI:10.1016/j.agwat.2018.12.015 |
| [12] |
Bremner J M. Recent research on problems in the use of urea as a nitrogen fertilizer[J]. Fertilizer Research, 1995, 42(1/2/3): 321-329. |
| [13] |
郑蕾, 王学东, 郭李萍, 等. 施肥对露地菜地氨挥发和氧化亚氮排放的影响[J]. 应用生态学报, 2018, 29(12): 4063-4070. ZHENG Lei, WANG Xue-dong, GUO Li-ping, et al. Impact of fertilization on ammonia volatilization and N2O emissions in an open vegetable field[J]. Chinese Journal of Applied Ecology, 2018, 29(12): 4063-4070. |
| [14] |
Mazzei L, Cianci M, Contaldo U, et al. Urease inhibition in the presence of N-(n-Butyl)thiophosphoric triamide, a suicide substrate:Structure and kinetics[J]. Biochemistry, 2017, 56(40): 5391-5404. DOI:10.1021/acs.biochem.7b00750 |
| [15] |
Sumner J B. The isolation and crystallization of the enzyme urease[J]. Journal of Biological Chemistry, 1926, 69: 435-441. |
| [16] |
Blakeley R L, Zerner B. Jack bean urease:The first nickel enzyme[J]. Journal of Molecular Catalysis, 1984, 23(2/3): 263-292. |
| [17] |
Mobley H L T, Hausinger R P. Microbial ureases:Significance, regulation, and molecular characterization[J]. Microbiological Reviews, 1989, 53(1): 85-108. DOI:10.1128/MMBR.53.1.85-108.1989 |
| [18] |
Mobley H L T, Island M D, Hausinger R P, et al. Molecular biology of microbial urease[J]. Microbiological Reviews, 1995, 59(3): 451-480. DOI:10.1128/MMBR.59.3.451-480.1995 |
| [19] |
Vallee B L, Auld D S. Active-site zinc ligands and activated H2O of zinc enzymes[J]. Proceedings of the National Academy of Sciences of the United States of America, 1990, 87(1): 220-224. DOI:10.1073/pnas.87.1.220 |
| [20] |
Burne R A, Chen Y Y. Bacterial ureases in infectious diseases[J]. Microbes Infect, 2000, 2(5): 533-542. |
| [21] |
Hastings R C, Saunders J R, Hall G H, et al. Application of molecular biological techniques to a seasonal study of ammonia oxidation in a eutrophic freshwater lake[J]. Applied and Environmental Microbiology, 1998, 64(10): 3674-3682. DOI:10.1128/AEM.64.10.3674-3682.1998 |
| [22] |
Mccaig A E, Phillips C J, Stephen J R, et al. Nitrogen cycling and community structure of proteobacterial β-subgroup ammonia-oxidizing bacteria within polluted marine fish farm sediments[J]. Applied and Environmental Microbiology, 1999, 65(1): 213-220. |
| [23] |
Allison S M, Prosser J I. Ammonia oxidation at low pH by attached populations of nitrifying bacteria[J]. Soil Biology and Biochemistry, 1993, 25(7): 935-941. |
| [24] |
Lehtovirta-Morley L E, Stoecker K, Vilcinskas A, et al. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(38): 15892-15897. DOI:10.1073/pnas.1107196108 |
| [25] |
Stahl D A, de la Torre J R. Physiology and diversity of ammonia-oxidizing archaea[J]. Annual Review of Microbiology, 2012, 66: 83-101. DOI:10.1146/annurev-micro-092611-150128 |
| [26] |
Hallam S J, Mincer T J, Schleper C, et al. Pathways of carbon assimilation and ammonia oxidation suggested by environmental genomic analyses of marine Crenarchaeota[J]. PLoS Biology, 2006, 4(4): 520-536. |
| [27] |
Shen J P, Zhang L M, Di H J, et al. A review of ammonia-oxidizing bacteria and archaea in Chinese soils[J]. Frontiers in Microbiology, 2012, 3: 296. |
| [28] |
Klotz M G, Norton J M. Sequence of an ammonia monooxygenase subunit A-encoding gene from Nitrosospira sp. NpAV[J]. Gene, 1995, 163(1): 159-160. |
| [29] |
Klotz M G, Norton J M. Multiple copies of ammonia monooxygenase (amo)operons have evolved under biased AT/GC mutational pressure in ammonia-oxidizing autotrophic bacteria[J]. FEMS Microbiology Letters, 1998, 168(2): 303-311. |
| [30] |
Alzerreca J J, Norton J M, Klotz M G. The amo operon in marine, ammonia-oxidizing γ-proteobacteria[J]. FEMS Microbiology Letters, 1999, 180(1): 21-29. |
| [31] |
Arp D J, Sayavedra-Soto L A, Hommes N G. Molecular biology and biochemistry of ammonia oxidation by Nitrosomonas europaea[J]. Archives of Microbiology, 2002, 178(4): 250-255. |
| [32] |
Sundermeyer-Klinger H, Meyer W, Warninghoff B, et al. Membranebound nitrate oxidoreductase of nitrobacter:Evidence for a nitrate reductase system[J]. Archives of Microbiology, 1984, 140(2/3): 153-158. |
| [33] |
Hochstein L I, Tomlinson G A, Hochstein L I. The enzymes associated with denitrification[J]. Archives Review of Microbiology, 1988, 42: 231-261. DOI:10.1146/annurev.mi.42.100188.001311 |
| [34] |
Daims H, Lebedeva E V, Pjevac P, et al. Complete nitrification by Nitrospira bacteria[J]. Nature, 2015, 528(7583): 504-509. |
| [35] |
Pinto A J, Marcus D N, Zeeshan lija U, et al. Metagenomic evidence for the presence of comammox Nitrospira-like bacteria in a drinking water system[J]. mSphere, 2015. DOI:10.1128/mSphere.00054-15 |
| [36] |
Daims H, Lücker S, Wagner M. A new perspective on microbes formerly known as nitrite-oxidizing bacteria[J]. Trends in Microbiology, 2016, 24(9): 699-712. DOI:10.1016/j.tim.2016.05.004 |
| [37] |
Maixner F, Noguera D R, Anneser B, et al. Nitrite concentration influences the population structure of Nitrospira-like bacteria[J]. Environmental Microbiology, 2006, 8(8): 1487-1495. DOI:10.1111/j.1462-2920.2006.01033.x |
| [38] |
Xu S Y, Wang B Z, Li Y, et al. Ubiquity, diversity, and activity of comammox Nitrospira in agricultural soils[J]. Science of the Total Environment, 2020, 706: 135684. DOI:10.1016/j.scitotenv.2019.135684 |
| [39] |
Liu S, Han P, Hink L, et al. Abiotic conversion of extracellular NH2OH contributes to N2O emission during ammonia oxidation[J]. Environmental Science & Technology, 2017, 51(2): 13122-13132. |
| [40] |
Daims H, Nielsen J L, Nielsen P H, et al. In situ characterization of Nitrospira-like nitrite-oxidizing bacteria active in wastewater treatment plants[J]. Applied Environmental Microbiology, 2001, 67(11): 5273-5284. DOI:10.1128/AEM.67.11.5273-5284.2001 |
| [41] |
Barth G, Otto R, Ferraz-Almeida R, et al. Conversion of ammonium to nitrate and abundance of ammonium-oxidizing-microorganism in tropical soils with nitrification inhibitor[J]. Scientia Agricola, 2020, 77(4): 1-5. |
| [42] |
Pedrazzini F, Tarsitano R, Nannipieri P. The effect of phenyl phosphorodiamidate on urease activity and ammonia volatilization in flooded rice[J]. Biology and Fertility of Soils, 1987, 3(3): 183-188. DOI:10.1007/BF00255781 |
| [43] |
Jabri E, Carr M B, Hausinger R P, et al. The crystal structure of urease from Klebsiella aerogenes[J]. Science, 1995, 268(5213): 998-1004. DOI:10.1126/science.7754395 |
| [44] |
Krajewska B, Brindell M. Urease activity and L-ascorbic acid[J]. Journal of Enzyme Inhibition and Medicinal Chemistry, 2010, 26(3): 309-318. |
| [45] |
Todd M J, Hausinger R R. Competitive inhibitors of Klebsiella aerogenes urease:Mechanisms of interaction with the nickel active site[J]. The Journal of Biological Chemistry, 1989, 264(27): 15835-18842. |
| [46] |
Zaborska W, Krajewska B, Kot M, et al. Quinone-induced inhibition of urease:Elucidation of its mechanisms by probing thiol groups of the enzyme[J]. Bioorganic Chemistry, 2007, 35(3): 233-242. DOI:10.1016/j.bioorg.2006.11.001 |
| [47] |
Andrews R K, Dexter A, Blakeley R L, et al. Jack bean urease(EC 3. 5. 1. 5)Ⅷ:On the inhibition of urease by amides and esters of phosphoric acid[J]. Journal of the American Chemical Society, 1986, 108(22): 7124-7125. |
| [48] |
Watson C J, Miller H, Poland P, et al. Soil properties and the ability of the urease inhibitor N-(n-BUTYL) thiophosphoric triamide (nBTPT)to reduce ammonia volatilization from surface-applied urea[J]. Soil Biology and Biochemistry, 1994, 26(9): 1165-1171. |
| [49] |
Krajewska B, Zaborska W, Leszko M. Inhibition of chitosan-immobilized urease by slow-binding inhibitors:Ni2+, F- and acetohydroxamic acid[J]. Journal of Molecular Catalysis B:Enzymatic, 2001, 14(4/5/6): 101-109. |
| [50] |
Zerner B. Recent advances in the chemistry of an old enzyme, urease[J]. Bioorganic Chemistry, 1991, 19(1): 116-131. |
| [51] |
Krajewska B, Piwowarska Z. Free vs chitosan-immobilized urease:Microenvironmental effects on enzyme inhibitions[J]. Biocatalysis and Biotransformation, 2005, 23(3/4): 225-232. |
| [52] |
Leopoldini M, Marino T, Russo N, et al. On the binding mode of urease active site inhibitors:A density functional study[J]. International Journal of Quantum Chemistry, 2008, 108(11): 2023-2029. |
| [53] |
Trenkel M E. Controlled-release and stabilized fertilizers in agriculture[M]. Paris: The International Fertilizer Industry Association, 1997: 1-151.
|
| [54] |
Rajbanshi S S, Benckiser G, Ottow J C G. Effects of concentration, incubation temperature, and repeated applications on degradation kinetics of dicyandiamide(DCD)in model experiments with a silt loam soil[J]. Biology and Fertility of Soils, 1992, 13(2): 61-64. DOI:10.1007/BF00337336 |
| [55] |
Zerulla W, Barth T, Dressel J, et al. 3, 4-dimethylphyrazole phosphate(DMPP):A new nitrification inhibitor for agriculture and horticulture[J]. Biology and Fertility of Soils, 2001, 34(2): 79-84. DOI:10.1007/s003740100380 |
| [56] |
Ruser R, Schulz R. The effect of nitrification inhibitors on the nitrous oxide(N2O) release from agricultural soils:A review[J]. Journal of Plant Nutrition and Soil Science, 2015, 178(2): 171-188. DOI:10.1002/jpln.201400251 |
| [57] |
Bending G D, Lincoln S D. Inhibition of soil nitrifying bacteria communities and their activities by glucosinolate hydrolysis products[J]. Soil Biology and Biochemistry, 2000, 32(8/9): 1261-1269. |
| [58] |
Bédard C, Knowles R. Physiology, biochemistry, and specific inhibitor of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers[J]. Microbiological Reviews, 1989, 53(1): 68-84. DOI:10.1128/MMBR.53.1.68-84.1989 |
| [59] |
Singh S N, Verma A. The potential of nitrification inhibitors to manage the pollution effect of nitrogen fertilizers in agricultural and other soils:A review[J]. Environmental Practice, 2007, 9(4): 266-279. DOI:10.1017/S1466046607070482 |
| [60] |
Keener W K, Arp D J. Kinetic studies of ammonia monooxygenase inhibition in Nitrosomonas europaea by hydrocarbons and halogenated hydrocarbons in an optimized whole-cell assay[J]. Applied and Environmental Microbiology, 1993, 59(8): 2501-2510. DOI:10.1128/AEM.59.8.2501-2510.1993 |
| [61] |
Arp D J, Stein L Y. Metabolism of inorganic N compounds by ammonia-oxidizing bacteria[J]. Critical Reviews in Biochemistry and Molecular Biology, 2003, 38(6): 471-495. |
| [62] |
Hyman M R, Arp D J. 14C2H2-and 14CO2-labeling studies of the de novo synthesis of polypeptides by Nitrosomonas europaea during recovery from acetylene and light inactivation of ammonia monooxygenase[J]. Journal of Biological Chemistry, 1992, 267(3): 1534-1545. |
| [63] |
Juliette L Y, Hyman M R, Arp D J. Mechanism-based inactivation of ammonia monooxygenase in Nitrosomonas europaea by allylsulfide[J]. Applied and Environmental Microbiology, 1993, 59(11): 3728-3735. DOI:10.1128/AEM.59.11.3728-3735.1993 |
| [64] |
Hyman M R, Russell S A, Ely R L, et al. Inhibition, inactivation, and recovery of ammonia-oxidizing activity in cometabolism of trichloroethylene by Nitrosomonas europaea[J]. Applied and Environmental Microbiology, 1995, 61(4): 1480-1487. DOI:10.1128/AEM.61.4.1480-1487.1995 |
| [65] |
Subbarao G V, Kishii M, Nakahara K, et al. Biological nitrification inhibition(BNI):Is there potential for genetic interventions in the Triticeae?[J]. Breeding Science, 2009, 59(5): 529-545. DOI:10.1270/jsbbs.59.529 |
| [66] |
Leininger S, Urich T, Schloter M, et al. Archaea predominate among ammonia-oxidizing prokaryotes in soils[J]. Nature, 2006, 442(7104): 806-809. DOI:10.1038/nature04983 |
| [67] |
Subbarao G V, Ishikawa T, Ito O, et al. A bioluminescence assay to detect nitrification inhibitors released from plant roots:A case study with Brachiaria humidicola[J]. Plant and Soil, 2006, 288(1/2): 101-112. |
| [68] |
Subbarao G V, Nakahara K, Hurtado M P, et al. Evidence for biological nitrification inhibition in Brachiaria pastures[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(41): 17302-17307. DOI:10.1073/pnas.0903694106 |
| [69] |
Coskun D, Britto D T, Shi W M, et al. How plant root exudates shape the nitrogen cycle[J]. Trends in Plant Science, 2017, 22(8): 661-673. DOI:10.1016/j.tplants.2017.05.004 |
| [70] |
Zakir H A, Subbarao G V, Pearse S J, et al. Detection, isolation and characterization of a root-exuded compound, methyl 3-(4-hydroxyphenyl)propionate, responsible for biological nitrification inhibition by sorghum(Sorghum bicolor)[J]. New Phytologist, 2008, 180(2): 442-451. DOI:10.1111/j.1469-8137.2008.02576.x |
| [71] |
Subbarao G V, Nakahara K, Ishikawa T, et al. Biological nitrification inhibition(BNI)activity in sorghum and its characterization[J]. Plant and Soil, 2013, 366(1/2): 243-259. |
| [72] |
De Boer W, Kowalchuk G A. Nitrification in acid soils:Microorganisms and mechanisms[J]. Soil Biology and Biochemistry, 2001, 33(7/8): 853-866. |
| [73] |
Zhang X N, Lu Y F, Yang T, et al. Factors influencing the release of the biological nitrification inhibitor 1, 9-decanediol from rice(Oryza sativa L.)roots[J]. Plant Soil, 2019, 436: 253-265. DOI:10.1007/s11104-019-03933-1 |
| [74] |
Sun L, Lu Y F, Yu F W, et al. Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency[J]. New Phytologist, 2016, 212(3): 646-656. DOI:10.1111/nph.14057 |
| [75] |
王国强, 孙焕明, 彭婧, 等. 生物硝化抑制剂应用研究进展[J]. 安徽农业科学, 2016, 44(11): 66-69, 78. WANG Guo-qiang, SUN Huan-ming, PENG Jing, et al. Research progress of the application of biological nitrification inhibitors[J]. Journal of Anhui Agricultural Sciences, 2016, 44(11): 66-69, 78. DOI:10.3969/j.issn.0517-6611.2016.11.023 |
| [76] |
Xia W W, Zhang C X, Zeng X W, et al. Autotrophic growth of nitrifying community in an agricultural soil[J]. The ISME Journal, 2011, 5: 1226-1236. DOI:10.1038/ismej.2011.5 |
| [77] |
Chen H, Chang Y, Fan X P, et al. Reduction of N2O emission by biochar and/or 3, 4-dimethylpyrazole phosphate(DMPP) is closely linked to soil ammonia oxidizing bacteria and nosZI-N2O reducer populations[J]. Science of the Total Environment, 2019, 694: 133658. DOI:10.1016/j.scitotenv.2019.133658 |
| [78] |
Duan P P, Zhang Q Q, Zhang X, et al. Mechanisms of mitigating nitrous oxide emissions from vegetable soil varied with manure, biochar and nitrification inhibitors[J]. Agricultural and Forest Meteorology, 2019, 278: 107672. DOI:10.1016/j.agrformet.2019.107672 |
| [79] |
Di H J, Cameron K C, Shen J P, et al. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils[J]. Nature Geoscience, 2009, 2: 621-624. DOI:10.1038/ngeo613 |
| [80] |
O' Callaghan M, Gerard E M, Carter P E, et al. Effect of the nitrification inhibitor dicyandiamide(DCD)on microbial communities in a pasture soil amended with bovine urine[J]. Soil Biology and Biochemistry, 2010, 42(9): 1425-1436. |
| [81] |
Di H J, Cameron K C. Inhibition of ammonium oxidation by a liquid formulation of 3, 4-dimethylpyrazole phosphate(DMPP) compared with a dicyandiamide(DCD) solution in six New Zealand grazed grassland soils[J]. J Soils Sediments, 2011, 11: 1032-1039. DOI:10.1007/s11368-011-0372-1 |
| [82] |
Gong P, Zhang L L, Wu Z J, et al. Response of ammonia-oxidizing bacteria and archaea in two agricultural soils to nitrification inhibitors DCD and DMPP:A pot experiment[J]. Pedosphere, 2013, 23(6): 729-739. |
| [83] |
Di H J, Cameron K C, Podolyan A, et al. Effect of soil moisture status and a nitrification inhibitor, dicyandiamide, on ammonia oxidizer and denitrifier growth and nitrous oxide emissions in a grassland soil[J]. Soil Biology and Biochemistry, 2014, 73: 59-68. DOI:10.1016/j.soilbio.2014.02.011 |
| [84] |
Dong D, Kou Y P, Yang W C, et al. Effects of urease and nitrification inhibitors on nitrous oxide emissions and nitrifying/denitrifying microbial communities in a rainfed maize soil:A 6-year field observation[J]. Soil & Tillage Research, 2018, 180: 82-90. |
| [85] |
Akiyama H, Yan X Y, Yagi K. Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils:Meta-analysis[J]. Global Change Biology, 2010, 16(6): 1837-1846. |
| [86] |
Zhang L M, Hu H W, Shen J P, et al. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils[J]. The ISME Journal, 2012, 6(5): 1032-1045. DOI:10.1038/ismej.2011.168 |
| [87] |
陈晨.田间管理措施对菜地N2O排放及功能基因丰度的影响研究[D].南京: 南京农亚大学, 2017: 42-43. CHEN Chen. Influence of field managements on N2O emissions and the abundance of N-cycling related functional genes in vegetable field[D]. Nanjing: Nanjing Agricultural University, 2017: 42-43. |
| [88] |
Cassman N A, Soares J R, Pijl A, et al. Nitrification inhibitors effectively target N2O-producing Nitrosospira spp. in tropical soil[J]. Environmental Microbiology, 2019, 21(4): 1241-1254. |
| [89] |
Corrochano-Monsalve M, Huérfano X, Menéndez S, et al. Relationship between tillage management and DMPSA nitrification inhibitor efficiency[J]. Science of the Total Environment, 2019, 134748. DOI:10.1016/j.scitotenv.2019.134748 |
| [90] |
Fisk L M, Maccarone L D, Barton L, et al. Nitrapyrin decreased nitrification of nitrogen released from soil organic matter but not amoA gene abundance at high soil temperature[J]. Soil Biology and Biochemistry, 2015, 88: 214-223. DOI:10.1016/j.soilbio.2015.05.029 |
| [91] |
Xi R J, Long X E, Huang S, et al. pH rather than nitrification and urease inhibitors determines the community of ammonia oxidizers in a vegetable soil[J]. AMB Express, 2017, 7: 129. DOI:10.1186/s13568-017-0426-x |
| [92] |
刘涛.氯甲基吡啶对石灰性土壤N2O排放的影响及其微生物作用机制[D].石河子: 石河子大学, 2018: 51-52. LIU Tao. Effect of nitrapyrin on N2O emissions and its microbial mechanism in calcareous soil[D]. Shihezi: Shihezi University, 2018: 51-52. |
| [93] |
Offre P, Prosser J I, Nicol GW. Growth of ammonia-oxidizing archaea in soil microcosms is inhibited by acetylene[J]. FEMS Microbiology Ecology, 2009, 70(1): 99-108. |
| [94] |
Zhang L M, Offre P R, He J Z, et al. Autotrophic ammonia oxidation by soil thaumarchaea[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(40): 17240-17245. DOI:10.1073/pnas.1004947107 |
| [95] |
Li C Y, Hu H W, Chen Q L, et al. Comammox Nitrospira play an active role in nitrification of agricultural soils amended with nitrogen fertilizers[J]. Soil Biology and Biochemistry, 2019, 138: 107609. DOI:10.1016/j.soilbio.2019.107609 |
| [96] |
Li W Y, Xiao Q, Hu C S, et al. A comparison of the efficiency of different urease inhibitors and their effects on soil prokaryotic community in a short-term incubation experiment[J]. Geoderma, 2019, 354: 113877. DOI:10.1016/j.geoderma.2019.07.035 |
| [97] |
Sanz-Cobena A, Sánchez-martín L, García-Torres L, et al. Gaseous emissions of N2O and NO and NO3- leaching from urea applied with urease and nitrification inhibitors to a maize(Zea mays)crop[J]. Agriculture, Ecosystems and Environment, 2012, 149: 64-73. |
| [98] |
Fan C H, Li B, Xiong Z Q. Nitrification inhibitors mitigated reactive gaseous nitrogen intensity in intensive vegetable soils from China[J]. Science of the Total Environment, 2017, 612: 480-489. |
| [99] |
Guardia G, Cangani M T, Andreu G, et al. Effect of inhibitors and fertigation strategies on GHG emissions, NO fluxes and yield in irrigated maize[J]. Field Crops Research, 2017, 204: 135-145. DOI:10.1016/j.fcr.2017.01.009 |
| [100] |
Ti C P, Xia L L, Chang S X, et al. Potential for mitigating global agricultural ammonia emission:A meta-analysis[J]. Environmental Pollution, 2019, 245: 141-148. |
| [101] |
Pan B B, Lam S K, Mosier A, et al. Ammonia volatilization from synthetic fertilizers and its mitigation strategies:A global synthesis[J]. Agriculture, Ecosystems and Environment, 2016, 232: 283-289. DOI:10.1016/j.agee.2016.08.019 |
| [102] |
Qiao C L, Liu L L, Hu S J, et al. How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input[J]. Global Change Biology, 2015, 21(3): 1249-1257. DOI:10.1111/gcb.12802 |
| [103] |
Akiyama H, Tsuruta H, Watanabe T. N2O and NO emissions from soils after the application of different chemical fertilizers[J]. Chemosphere-Global Change Science, 2000, 2(3/4): 313-320. |
| [104] |
Guardia G, Sanz-Cobena A, Sanchez-Martín L. Urea-based fertilization strategies to reduce yield-scaled N oxides and enhance bread-making quality in a rainfed Mediterranean wheat crop[J]. Agriculture, Ecosystems and Environment, 2018, 265: 421-431. DOI:10.1016/j.agee.2018.06.033 |
| [105] |
徐玉秀, 郭李萍, 谢立勇, 等. 中国主要旱地农田N2O背景排放量及排放系数特点[J]. 中国农业科学, 2016, 49(9): 1729-1743. XU Yu-xiu, GUO Li-ping, XIE Li-yong, et al. Characteristics of background emissions and emission factors of N2O from major upland fields in China[J]. Scientia Agricultura Sinica, 2016, 49(9): 1729-1743. |
| [106] |
Thapa R, Awale R, McGranahan D A. Effect of enhanced efficiency fertilizers on nitrous oxide emissions and crop yields:A meta-analysis[J]. Soil Science Society of America Journal, 2016, 80(5): 1121-1134. DOI:10.2136/sssaj2016.06.0179 |
| [107] |
Jiang J Y, Fan Hui, Pang B K, et al. Assessment of reactive nitrogen mitigation potential of different nitrogen treatments under directseeded rice and wheat cropping system[J]. Environmental Science and Pollution Research, 2018, 25: 20241-20254. DOI:10.1007/s11356-018-2104-1 |
| [108] |
孙祥鑫, 李东坡, 武志杰, 等. 持续施用缓/控释尿素条件下水田土壤NH3挥发与N2O排放特征[J]. 应用生态学报, 2016, 27(6): 1901-1909. SUN Xiang-xin, LI Dong-po, WU Zhi-jie, et al. Characteristics of ammonia volatilization and nitrous oxide emission from a paddy soil under continuous application of different slow/controlled release urea[J]. Chinese Journal of Applied Ecology, 2016, 27(6): 1901-1909. |
| [109] |
Pawlick A, Wagner-Riddle C, Parkin G W, et al. Assessment of nitrification and urease inhibitors on nitrate leaching in corn(Zea mays L.)[J]. Canadian Journal of Soil Science, 2019, 99(1): 80-91. |
| [110] |
Recio J, Alvarez J M, Rodriguez-Quijano M, et al. Nitrifification inhibitor DMPSA mitigated N2O emission and promoted NO sink in rainfed wheat[J]. Environmental Pollution, 2019, 245: 199-207. DOI:10.1016/j.envpol.2018.10.135 |
| [111] |
Lai C M, Tabatabai M A. Kinetic parameters of immobilized urease[J]. Soil Biology and Biochemistry, 1992, 24(3): 225-228. DOI:10.1016/0038-0717(92)90222-J |
| [112] |
Carmona G, Christianson C B, Byrnes B H. Temperature and low concentration effects of the urease inhibitor N-(n-butyl)thiophosphoric triamide(nBTPT)on ammonia volatilization from urea[J]. Soil Biology and Biochemistry, 1990, 22(7): 933-937. |
| [113] |
隽英华, 陈利军, 武志杰. 温度和脲酶抑制剂影响下的土壤脲酶动力学行为[J]. 辽宁工程技术大学学报(自然科学版), 2009, 28(6): 1021-1025. JUAN Ying-hua, CHEN Li-jun, WU Zhi-jie. Kinetic behaviors of soil urease under influence of temperature and urease inhibitors[J]. Journal of Liaoning Technical University(Natural Science), 2009, 28(6): 1021-1025. DOI:10.3969/j.issn.1008-0562.2009.06.042 |
| [114] |
周旋, 吴良欢, 戴锋. 土壤温度和含水量互作对抑制剂抑制氮素转化效果的影响[J]. 农业工程学报, 2017, 33(20): 106-115. ZHOU Xuan, WU Liang-huan, DAI Feng. Inhibition effect of inhibitors on nitrogen transformation affected by interaction of soil temperature and water content[J]. Transactions of the Chinese Society of Agricultural Engineering, 2017, 33(20): 106-115. DOI:10.11975/j.issn.1002-6819.2017.20.014 |
| [115] |
Bremner J M, Chai H S. Evaluation of N-butyl phosphorothioic triamide for retardation of urea hydrolysis in soil[J]. Communications in Soil Science and Plant Analysis, 1986, 17(3): 337-351. DOI:10.1080/00103628609367716 |
| [116] |
Watson C J, Akhonzada N A, Hamilton J T G, et al. Rate and mode of application of the urease inhibitor N -(n-butyl)thiophosphoric triamide on ammonia volatilization from surface-applied urea[J]. Soil Use and Management, 2008, 24(3): 246-253. DOI:10.1111/j.1475-2743.2008.00157.x |
| [117] |
Kleineidam K, Košmrlj K, Kublik S, et al. Influence of the nitrification inhibitor 3, 4-dimethylpyrazole phosphate(DMPP)on ammonia-oxidizing bacteria and archaea in rhizosphere and bulk soil[J]. Chemosphere, 2011, 84(1): 182-186. DOI:10.1016/j.chemosphere.2011.02.086 |
| [118] |
Menéndez S, Barrena I, Setien I, et al. Efficiency of nitrification inhibitor DMPP to reduce nitrous oxide emissions under different temperature and moisture conditions[J]. Soil Biology and Biochemistry, 2012, 53: 82-89. DOI:10.1016/j.soilbio.2012.04.026 |
| [119] |
Chen Q H, Qi L Y, Bi Q F, et al. Comparative effects of 3, 4-dimethylpyrazole phosphate(DMPP)and dicyandiamide(DCD)on ammonia-oxidizing bacteria and archaea in a vegetable soil[J]. Applied Microbiology and Biotechnology, 2015, 99(1): 477-487. |
| [120] |
殷建祯, 俞巧钢, 符建荣, 等. 不同作用因子下有机无机配施添加DMPP对氮素转化的影响[J]. 土壤学报, 2013, 50(3): 574-583. YIN Jian-zhen, YU Qiao-gang, FU Jian-rong, et al. Effects of combined application of organic and inorganic fertilizers plus DMPP on nitrogen transformation in soils as affected by different factors[J]. Acta Pedologica Sinica, 2013, 50(3): 574-583. |
| [121] |
Faria L D A, Nascimento C A C D, Bardella G R, et al. NH3 volatilization from urea-NBPT in Eucalyptus[J]. Communications in Soil Science and Plant Analysis, 2016, 47(6): 769-774. DOI:10.1080/00103624.2016.1146892 |
| [122] |
Sanz-Cobena A, Misselbrook T, Camp V, et al. Effect of water addition and the urease inhibitor NBPT on the abatement of ammonia emission from surface applied urea[J]. Atmospheric Environment, 2011, 45(8): 1517-1524. |
| [123] |
Sanz-Cobena A, Abalos D, Meijide A, et al. Soil moisture determines the effectiveness of two urease inhibitors to decrease N2O emission[J]. Mitigation and Adaptation Strategies for Global Change, 2016, 21(7): 1131-1144. |
| [124] |
Briggs G G. The behaviour of the nitrification inhibitor "N-serve" in broadcast and incorporated applications to soil[J]. Journal of the Science of Food and Agriculture, 1975, 26(8): 1083-1092. |
| [125] |
Puttanna K, Nanje Gowda N M, Prakasa Rao E V S. Effect of concentration, temperature, moisture, liming and organic matter on the efficacy of the nitrification inhibitors benzotriazole, o-nitrophenol, m-nitroaniline and dicyandiamide[J]. Nutrient Cycling in Agroecosystems, 1999, 54(3): 251-257. DOI:10.1023/A:1009826927579 |
| [126] |
史云峰, 赵牧秋, 张丽莉. 双氰胺(DCD)在砖红壤中硝化抑制效果的影响因素研究[J]. 安徽农业科学, 2011, 39(33): 20437-20440. SHI Yun-feng, ZHAO Mu-qiu, ZHANG Li-li. Research on the factors affecting nitrification inhibition of dicyandiamide(DCD)in Latosol[J]. Journal of Anhui Agricultural Sciences, 2011, 39(33): 20437-20440. DOI:10.3969/j.issn.0517-6611.2011.33.052 |
| [127] |
Engel R E, Towey B D, Gravens E, et al. Degradation of the urease inhibitor NBPT as affected by soil pH[J]. Soil Science Society of America Journal, 2015, 79(6): 1674-1683. DOI:10.2136/sssaj2015.05.0169 |
| [128] |
Engel R E, Jones C, Wallander R. Ammonia volatilization from urea and mitigation by NBPT following surface application to cold soils[J]. Soil Science Society of America Journal, 2011, 75(6): 2348-2357. DOI:10.2136/sssaj2011.0229 |
| [129] |
Fan X P, Yin C, Yan G C, et al. The contrasting effects of N-(n-butyl) thiophosphoric triamide(NBPT) on N2O emissions in arable soils differing in pH are underlain by complex microbial mechanisms[J]. Science of the Total Environment, 2018, 642: 155-167. DOI:10.1016/j.scitotenv.2018.05.356 |
| [130] |
Guo L P, Wang X D, Diao T T, et al. N2O emission contributions by different pathways and associated microbial community dynamics in a typical calcareous vegetable soil[J]. Environmental Pollution, 2018, 242(Part B): 2005-2013. |
| [131] |
Jiang X J, Hou X Y, Zhou X, et al. pH regulates key players of nitrification in paddy soils[J]. Soil Biology and Biochemistry, 2015, 81: 9-16. DOI:10.1016/j.soilbio.2014.10.025 |
| [132] |
赖晶晶, 兰婷, 王启, 等. 硝化抑制剂对紫色土硝化作用及N2O排放的影响[J]. 农业环境科学学报, 2019, 38(6): 1420-1428. LAI Jing-jing, LAN Ting, WANG Qi, et al. Effects of nitrification inhibitors on purple soil nitrification and N2O emissions[J]. Journal of Agro-Environment Science, 2019, 38(6): 1420-1428. |
| [133] |
周旋, 吴良欢, 戴锋. 新型磷酰胺类脲酶抑制剂对不同质地土壤尿素转化的影响[J]. 应用生态学报, 2016, 27(12): 4003-4012. ZHOU Xuan, WU Liang-huan, DAI Feng. Influence of a new phosphoramide urease inhibitor on urea-N transformation in different texture soil[J]. Chinese Journal of Applied Ecology, 2016, 27(12): 4003-4012. |
| [134] |
Skiba U, Ball B C. The effect of soil texture and soil drainage on emissions of nitric oxide and nitrous oxide[J]. Soil Use and Management, 2002, 18(1): 56-60. DOI:10.1079/SUM2002101 |
| [135] |
Rochette P. No-till only increases N2O emissions in poorly-aerated soils[J]. Soil and Tillage Research, 2008, 101(1/2): 97-100. |
| [136] |
Neufeld J D, Knowles R. Inhibition of nitrifiers and methanotrophs from an agricultural humisol by allylsulfide and its implications for environmental studies[J]. Applied and Environmental Microbiology, 1999, 65(6): 2461-2465. DOI:10.1128/AEM.65.6.2461-2465.1999 |
| [137] |
Barth G, von Tucher S, Schmidhalter U, et al. Performance of nitrification inhibitors with different nitrogen fertilizers and soil textures[J]. Journal of Plant Nutrition and Soil Sciences, 2019, 182(5): 694-700. DOI:10.1002/jpln.201800594 |
| [138] |
Wang Z P, Van Cleemput O, Li L T, et al. Effect of organic matter and urease inhibitors on urea hydrolysis and immobilization of urea nitrogen in an alkaline soil[J]. Biology and Fertility of Soils, 1991, 11(2): 101-104. DOI:10.1007/BF00336372 |
| [139] |
Suter H C, Pengthamkeerati P, Walker C, et al. Influence of temperature and soil type on inhibition of urea hydrolysis by N-(n-butyl) thiophosphoric triamide in wheat and pasture soils in south-eastern Australia[J]. Soil Research, 2011, 49(4): 315-319. DOI:10.1071/SR10243 |
| [140] |
Barth G, Tucher S V, Schmidhalter U. Influence of soil parameters on the efficiency of the new nitrification inhibitor DMPP(ENTECⓇ)[J]. Developments in Plant & Soil Sciences, 2001, 92: 756-757. |
| [141] |
Zhang H J, Wu Z J, Zhou Q X. Dicyandiamide sorption-desorption behavior on soils and peat humus[J]. Pedosphere, 2004, 14(3): 395-399. |
| [142] |
薛妍, 武志杰, 张丽莉, 等. 土壤含水量、pH及有机质对DMPP硝化抑制效果的影响[J]. 应用生态学报, 2012, 23(10): 2663-2669. XUE Yan, WU Zhi -jie, ZHANG Li -li, et al. Inhibitory effect of DMPP on soil nitrification as affected by soil moisture content, pH and organic matter[J]. Chinese Journal of Applied Ecology, 2012, 23(10): 2663-2669. |
| [143] |
Ouverney C C, Fuhrman J A. Marine planktonic archaea take up amino acids[J]. Applied and Environmental Microbiology, 2001, 66(11): 4829-4833. |
| [144] |
Qin W, Amin S A, Martens-Habbena W, et al. Marine ammonia-oxidizing archaeal isolates display obligate mixotrophy and wide ecotypic variation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(34): 12504-12509. DOI:10.1073/pnas.1324115111 |
| [145] |
Morales A, Cruells M, Roca A, et al. Treatment of copper flash smelter flue dusts for copper and zinc extraction and arsenic stabilization[J]. Hydrometallurgy, 2010, 105(1/2): 148-154. |
| [146] |
倪秀菊, 李玉中, 徐春英, 等. 土壤脲酶抑制剂和硝化抑制剂的研究进展[J]. 中国农学通报, 2009, 25(12): 145-149. NI Xiu-ju, LI Yu-zhong, XU Chun-ying, et al. Advance of research on urease inhibitor and nitrification inhibitor in soil[J]. Chinese Agricultural Science Bulletin, 2009, 25(12): 145-149. |
| [147] |
张文学, 王萍, 孙刚, 等. 脲酶抑制剂不同用量对土壤氮素供应的影响[J]. 中国土壤与肥料, 2018, 278(6): 38-44, 52. ZHANG Wen-xue, WANG Ping, SUN Gang, et al. Effects of urease inhibitor addition rate on nitrogen transformation in paddy soil[J]. Soils and Fertilizers Sciences in China, 2018, 278(6): 38-44, 52. |
| [148] |
Luo Q X, Freney J R, Keerthisinghe D G, et al. Inhibition of urease activity in flooded soils by phenylphosphorodiamidate and n-(n-butyl)thiophosphorictriamide[J]. Soil Biology and Biochemistry, 1994, 26(8): 1059-1065. |
| [149] |
Xia L L, Lam S K, Chen D L, et al. Can knowledge-based N management produce more staple grain with lower greenhouse gas emission and reactive nitrogen pollution? A meta-analysis[J]. Global Change Biology, 2017, 23(5): 1917-1925. DOI:10.1111/gcb.13455 |
| [150] |
Azam F, Benckiser G, Müller C, et al. Release, movement and recovery of 3, 4-dimethylpyrazole phosphate(DMPP), ammonium, and nitrate from stabilized nitrogen fertilizer granules in a silty clay soil under laboratory conditions[J]. Biology and Fertility of Soils, 2001, 34(2): 118-125. DOI:10.1007/s003740100384 |
| [151] |
Di H J, Cameron K C. How does the application of different nitrification inhibitors affect nitrous oxide emissions and nitrate leaching from cow urine in grazed pastures?[J]. Soil Use and Management, 2012, 28(1): 54-61. DOI:10.1111/j.1475-2743.2011.00373.x |
| [152] |
杨柳青, 季加敏, 巨晓棠. 硝化/脲酶抑制剂对石灰性潮土N2O减排效果及氮素转化的比较[J]. 农业环境科学学报, 2007, 36(3): 605-612. YANG Liu-qing, JI Jia-min, JU Xiao-tang. Effects of nitrification/urease inhibitors on mitigating N2O emission and transformation of N from calcareous fluvo -aquic soil[J]. Journal of Agro-Environment Science, 2007, 36(3): 605-612. |
| [153] |
Liu Y Y, Wang R L, Zhang P, et al. The nitrification inhibitor methyl 3-(4-hydroxyphenyl)propionate modulates root development by interfering with auxin signaling via the NO /ROS pathway in Arabidopsis[J]. Plant Physiology, 2016, 171: 1686-1703. DOI:10.1104/pp.16.00670 |
| [154] |
Sahrawat K L. Nitrification in some tropical soils[J]. Plant and Soil, 1982, 65(2): 281-286. |
 2020, Vol. 39
2020, Vol. 39