华北平原作为我国第二大平原,涵盖京津冀和鲁豫皖苏等多个省市[1],是我国最重要的农作物生产基地之一[2],其典型的种植制度是“冬小麦-夏玉米”轮作,轮作区耕地面积高达1.22×107 hm2,可为全国供应70%以上的小麦和近30%的玉米[3],具有“投入高、产量高、环境影响大”的特点[4]。近年来,化肥的大量投入使农田土壤生态系统服务功能和养分循环受到严重影响,生物多样性大幅降低[5]。据报道,单施化肥会降低土壤微生物群落的丰度和活性,并对其组成产生负面影响[6]。在华北平原集约化耕作条件下,有机物质(秸秆和畜禽粪便)的施用是重要的土壤管理措施,其不但可以为植物提供养分,而且可以通过补充有机质(SOM)来维持土壤肥力[7]。小麦、玉米除作为粮食作物外,其副产物——秸秆含有丰富的氮、磷、钾,还田利用后可为后茬作物提供生长所必需的营养元素,提高作物产量[8]。Lori等[9]研究表明,施用有机肥会显著增加土壤细菌丰度和酶活性。此外,与单施化肥相比,有机肥还可以提高土壤微生物群落的多样性[10]。随着化肥的持续施用,其对环境的负面影响逐渐显现,与此同时,为了缓解无机肥料大量施用导致的农田土壤质量下降,有机肥的应用得到不断发展,有机肥与化肥配合施用成为近年来肥料应用研究中较为活跃且发展最快的领域之一[11]。有机-无机肥配施具有速效和长效的优势,可以促进土壤微生物的生长繁殖[12],并对粮食生产具有显著的积极影响[13]。研究表明,有机-无机肥配施可以影响土壤微生物群落组成,进而影响土壤养分循环[14]和植物生长[15]。土壤微生物群落在有机物质分解中发挥重要作用,影响着营养物质的生物地球化学循环,对维持土壤功能至关重要[16]。此外,季节动态变化也是影响农业生态系统中土壤微生物群落的重要因素之一[17-19]。在小麦-玉米轮作体系中,小麦田土壤理化性质与微生物群落之间的相关性强于玉米田[20],取样时间对土壤微生物群落的影响远大于施肥制度,施肥效应在小麦田最为显著[21]。Zhao等[22]的研究同样阐明了轮作体系下施肥时间对土壤微生物的显著影响。因此,在开展轮作体系下土壤微生物群落变化的研究中应该将作物种类和环境条件均视为驱动土壤微生物群落变化的主要因素。
氮循环是土壤生态系统生物地球化学循环中的核心过程之一,在农田生态系统可持续发展方面起着举足轻重的作用[23]。土壤微生物发挥与氮循环有关的生态功能[24]。目前,学者们在有机-无机肥配施对农田土壤微生物影响方面已开展了大量研究工作,初步探明了农田土壤细菌群落的组成、多样性和结构等响应特征及其影响因素。但尚不清楚轮作体系下施肥方式和季节性波动如何影响土壤微生物群落的氮循环功能。因此,本研究选择单施化肥、化肥配施玉米秸秆和化肥配施有机肥3种不同施肥处理,采用16S rRNA基因高通量测序技术,基于生物信息学方法并结合PICRUSt功能预测分析,探究土壤细菌功能分类变化,进而明确氮循环过程功能基因的变化及其与土壤理化性质间的关系,以期为更好地揭示有机-无机肥配施过程中氮循环机制提供理论参考。
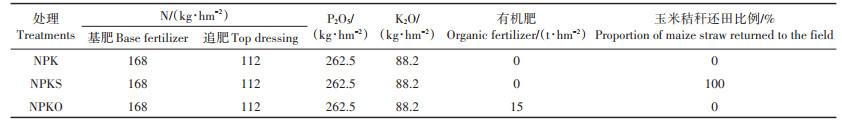
1 材料与方法 1.1 研究区概况与试验设计试验地位于天津市宁河区林场(39°48′ N,117° 71′E),该地农田耕作方式为华北农耕区常规耕作模式——冬小麦-夏玉米轮作。试验始于2015年,共设3个施肥处理:单施无机肥(NPK)、无机肥配施玉米秸秆(NPKS)和无机肥配施有机肥(NPKO)。各处理小区面积较大(22 m×22 m),故没有设置重复小区,取样时将小区分为3个区域即3次假重复[25-27]。共分小麦季和玉米季两次施肥,每次施肥各处理无机肥施用量相同(140 kg·hm-2 N,131.25 kg·hm-2 P2O5,44.1 kg· hm-2 K2O)。试验中施用的无机肥料包括无机复合肥(N含量28%,P2O5含量13%,K2O含量5%)、磷肥[Ca(H2PO4)2,养分含量12%]、钾肥(KCl,养分含量60%)以及尿素(N含量46%)。将168 kg∙hm−2 N(无机复合肥)作为基肥在冬小麦和夏玉米播种时一次性施入农田,112 kg·hm-2 N(尿素)在小麦苗期和玉米拔节期分别追施入农田。NPKO处理施用的有机肥(商品有机肥,养分总含量≥5%,有机质含量≥40%,石家庄市希星肥业科技有限公司生产)量为15 t·hm-2,只在小麦季一次性施入。各小区总施肥量见表 1。小麦季所有处理产生的小麦秸秆按当地常规耕作方式全部粉碎后覆盖于地表还田,玉米季节NPKS处理产生的玉米秸秆粉碎成小段后随土壤耕翻还田,NPK和NPKO处理的玉米秸秆均不还田。其他田间管理按照当地常规生产模式进行。
|
|
表 1 各试验小区总施肥量 Table 1 Fertilizer application rate in trail plots |
分别于2019年5月小麦收获前和9月玉米收获前,使用直径5 cm的土壤采样器采集0~20 cm耕层土壤,将在每个区域随机采集的5个土芯混合成1个土壤样品,剔除石砾和植物残体等杂质后装入灭菌自封袋暂存于保温箱中并迅速带回实验室,土壤样品过2 mm筛后用于提取DNA并测定土壤理化性质。
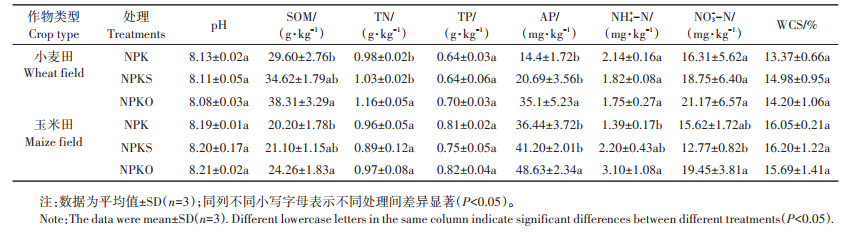
1.3 土壤理化性质测定土壤理化指标pH、有机质(SOM)、全氮(TN)、全磷(TP)、速效磷(AP)的测定方法参照文献[28],其中土壤pH在2.5:1的水土比下采用复合电极测定;SOM采用重铬酸钾容量法测定;TN采用半微量开氏法测定;TP采用HClO4-H2SO4消煮-钼锑抗比色法测定;AP采用NaHCO3浸提-钼锑抗比色法测定;硝态氮(NO3--N)和铵态氮(NH4+-N)经0.01 mol·L-1 CaCl2浸提后使用连续流动分析仪(AA3,SEAL Analytical,Germany)测定;将土壤样品于105 ℃下烘干至恒质量,采用重量法测定土壤含水量(WCS)。小麦田和玉米田土壤理化性质见表 2。
|
|
表 2 不同施肥处理土壤理化性质 Table 2 Physical and chemical properties of soil under different fertilization treatments |
采用Fast DNA® Spin Kit for Soil(MP Biomedicals,U.S.A)提取土壤DNA,经1%的琼脂糖凝胶电泳检测DNA质量后,使用NanoDrop2000测定DNA浓度和纯度。采用引物338F(5′ - ACTCCTACGGGAGGCAG- CAG-3′)和806R(5′ - GGACTACHVGGGTWTCTA- AT-3′)扩增细菌16S rRNA基因的V3~V4高变区序列[29],PCR条件如下:95 ℃预变性3 min,27个循环(95 ℃变性30 s,55 ℃退火30 s,72 ℃延伸45 s),然后72 ℃稳定延伸10 min。PCR反应体系包括:5×Trans- Start FastPfu缓冲液4 μL,2.5 mmol·L-1 dNTPs 2 μL,上下游引物(5 μmol·L-1)各0.8 μL,TransStart FastPfu DNA聚合酶1.0 U,模板DNA 10 ng,加ddH2O补足至20 μL。高通量测序委托上海美吉生物医药科技有限公司在Illumina MiSeq平台上完成。使用Trimmomatic软件对原始测序序列进行质控,并使用FLASH软件进行拼接。拼接时碱基重叠数不得少于20个,设置50 bp的窗口。根据PE序列之间的重叠关系,将成对序列拼接成一条序列,最小重叠长度为10 bp。拼接序列的重叠区允许的最大错配比率为0.2。根据序列首尾两端的条形码和引物区分样品,并调整序列方向,条形码允许的错配数为0,最大引物错配数为2[30]。使用UPARSE软件,根据97%的相似度将高质量的核酸序列聚类到操作分类单元(OTUs)并剔除嵌合体[31]。利用RDP classifier对每条序列进行物种分类注释,设置比对阈值为70%。
1.5 数据分析使用SPSS 23.0对细菌氮循环功能基因与土壤理化性质进行皮尔逊(Pearson)相关性分析,并利用单因素方差分析(One-way ANOVA)中的Tukey′ s方法对不同处理的土壤理化性质进行方差分析和显著性检验。使用Origin 2018绘制细菌氮循环功能基因丰度差异图,使用STAMP软件并采用Welch′s-test方法比较不同处理间细菌二级功能分类和三级功能分类中存在显著差异的功能丰度。
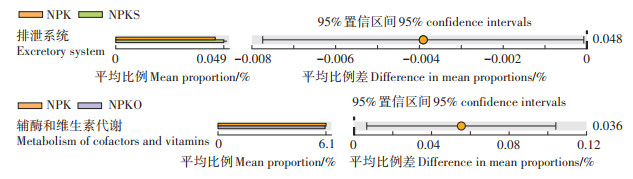
2 结果与讨论 2.1 土壤细菌功能分类基于KEGG数据对比样品中的细菌二级功能分类,NPKS、NPKO与NPK处理间存在显著差异的小麦土壤细菌功能丰度见图 1。与NPK处理相比,NPKS处理中排泄系统的相对丰度显著升高,升高幅度达到8.73%,而NPKO处理中辅酶和维生素代谢的相对丰度显著降低,降低幅度达到0.90%。NPKS、NPKO与NPK处理间玉米土壤细菌的功能丰度差异均不显著。
|
图 1 小麦土壤KEGG功能差异(二级功能分类) Figure 1 Differences of KEGG function in wheat soil(secondary functional classification) |
根据细菌三级功能分类,小麦土壤和玉米土壤NPKS、NPKO与NPK处理间相对丰度存在显著差异的细菌代谢功能见图 2。从整体来看,NPKS和NPK处理中,小麦土壤细菌氨基酸糖与核苷酸糖代谢(Amino sugar and nucleotide sugar metabolism)的相对丰度比其他代谢功能更大,其次是丙氨酸、天冬氨酸和谷氨酸代谢(Alanine,aspartate and glutamate metab⁃ olism)、硫胺素代谢(Thiamine metabolism)、脂多糖生物合成(Lipopolysaccharide biosynthesis)、核黄素代谢(Riboflavin metabolism)和长寿调节途径(Longevity regulating pathway-worm),这些功能的相对丰度均表现出NPKS处理显著低于NPK处理。与NPK处理相比,NPKO处理显著降低了Cell cycle-Caulobacter所占的相对丰度,但显著提高了碱基切除修复(Base excision repair)所占的相对丰度。NPKO处理中的硫胺素代谢和核黄素代谢所占的相对丰度均显著低于NPK处理。对于玉米土壤细菌,Glioma和神经营养素信号通路(Neurotrophin signaling pathway)在NPK处理中的相对丰度显著高于NPKS处理,而突触囊泡循环(Synaptic vesicle cycle)表现相反;甲烷代谢(Meth⁃ ane metabolism)在NPK和NPKO处理中存在显著差异。小麦季有机添加处理与无机处理下相对丰度具有显著差异的土壤细菌三级功能分类中的代谢功能个数(NPK与NPKS处理对比为12个,NPK与NPKO处理对比为10个)多于玉米季(NPK与NPKS处理对比为3个,NPK与NPKO处理对比为1个),表明有机添加对细菌代谢功能的影响在小麦土壤中发挥的作用更加复杂,这可能受两个取样时间的土壤理化性质不同所影响。这与先前的报道中小麦田土壤理化性质与土壤微生物群落之间的相关性强于玉米田相一致[20]。此外,有机肥于冬小麦种植时施入农田,且整个轮作体系下仅施入一次,冬小麦的生长周期较长且需越冬,气候变化强烈;而夏玉米的生长周期较短且气候炎热,降水量高。推测认为有机肥和气候条件差异同样可能造成细菌代谢功能的改变。
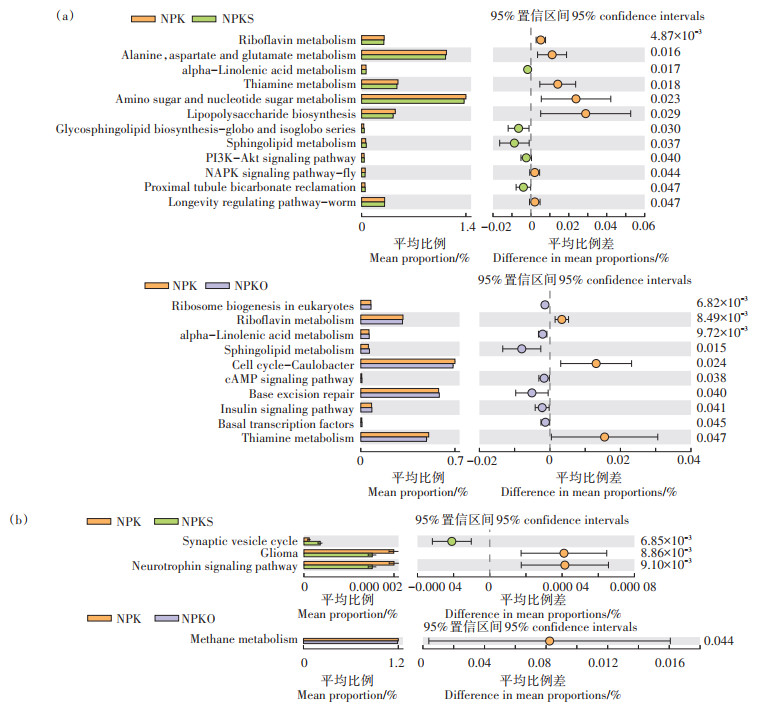
|
图 2 小麦土壤(a)和玉米土壤(b)KEGG功能差异(三级功能分类) Figure 2 Differences of KEGG function in wheat(a)and maize(b)soils(three-level functional classification) |
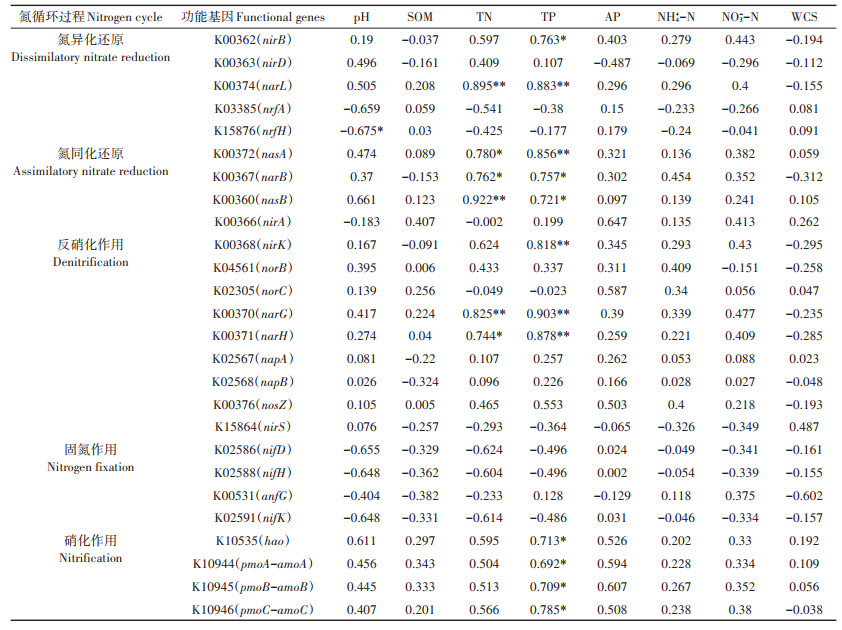
进一步研究有机-无机肥配施下麦玉轮作田细菌参与氮循环途径(K00910)的基因差异。根据氮循环中氮价位的变化,将氮循环过程分为5个阶段,分别是氮异化还原、氮同化还原、反硝化作用、固氮作用和硝化作用。氮异化还原过程由nirB、nirD、narL、nrfA和nrfH基因参与完成,氮同化还原过程由nasA、narB、nasB和nirA基因参与完成,反硝化作用由nirK、norB、norC、narG、narH、napA、napB、nosZ和nirS基因参与完成,固氮作用由nifD、nifH、anfG和nifK基因参与完成,硝化作用由hao、pmoA-amoA、pmoB-amoB和pmoC-amoC基因参与完成。
小麦和玉米土壤细菌氮循环相关基因的丰度变化如图 3所示。从整体来看,小麦和玉米土壤细菌的氮异化还原和氮同化还原潜力最高,反硝化潜力和固氮潜力次之,硝化潜力最弱。在小麦土壤细菌氮异化还原过程中,亚硝酸盐还原酶nirD基因最为丰富,NPK和NPKO处理中nirD基因丰度高于NPKS处理;其次是亚硝酸盐还原酶基因nirB和硝酸盐还原酶基因narL,NPKS和NPKO处理中nirB和narL基因丰度高于NPK处理;异化还原酶基因nrfA和nrfH所含基因丰度较低。氮同化还原过程中,硝酸盐还原酶基因nasA和nasB丰度高于narB和nirA,nasA和nasB基因丰度大小均表现为NPKO>NPKS>NPK。反硝化过程中,亚硝酸还原酶基因nirK、narH和硝酸还原酶基因narG丰度较高,大小同样表现为NPKO>NPKS>NPK;其次是一氧化氮还原酶基因norB和氧化亚氮还原酶的编码基因nosZ;丰度较低的基因是亚硝酸盐还原酶基因norC、异化还原酶基因napA和napB。固氮酶基因nifD、nifH和nifK在固氮作用中丰度较低。硝化作用基因hao、pmoA-amoA、pmoB-amoB和pmoC-amoC明显低于其他过程氮循环相关基因。玉米土壤细菌氮循环相关基因丰度高低整体上与小麦土壤细菌一致,但在各处理中的差异有所变化。氮异化还原过程中,NPK处理中nirD基因丰度高于NPKS和NPKO处理,NPK和NPKO处理中nirB和narL基因丰度高于NPKS处理。氮同化还原过程中,NPK和NPKO处理中nasA和nasB基因丰度高于NPKS处理。反硝化过程中,NPK和NPKO处理中亚硝酸还原酶基因nirK、narH和硝酸还原酶基因narG丰度高于NPKS处理。固氮酶基因nifD、nifH和nifK在NPKS处理中的丰度高于NPK和NPKO处理。
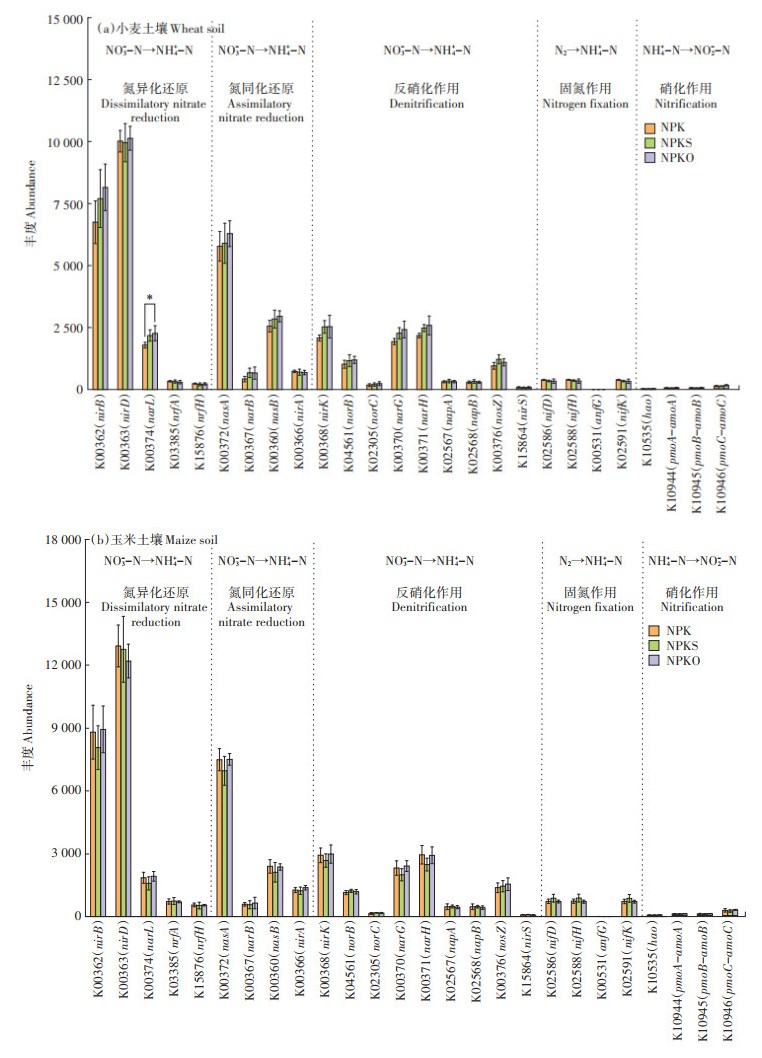
|
*表示在0.05水平上显著相关 * indicates a significant correlation at the level of 0.05 图 3 小麦土壤和玉米土壤细菌氮循环途径 Figure 3 Bacteria nitrogen cycling pathway in wheat and maize soil |
在小麦土壤细菌中,与单施化肥相比,配施秸秆和有机肥提高了反硝化基因丰度,这与前人的研究结果相一致[23, 32]。反硝化微生物大多是异养微生物,有机物料可以为土壤提供更均衡和稳定的营养,促进反硝化微生物的生长繁殖[23]。尽管氮循环主要由土壤微生物驱动,但是植物可以通过释放根系分泌物来控制根际及其附近的微生物群体催化氮转化[33]。根系分泌物主要对氮循环过程中的硝化作用和固氮作用起控制作用[34]。不同取样时间,不同农田种植作物,小麦和玉米根际分泌物可能成为影响氮循环功能基因丰度变化的制约因素之一。例如,小麦根系分泌物可以释放生物硝化抑制剂来控制土壤硝化作用[35],但在玉米根系中并没有发现生物硝化抑制作用[36]。此外,有报道表明高温条件下,一些生态系统的根系分泌物增强,这可能导致与土壤有机质分解和根际氮周转相关的微生物活动加速[37]。因此,小麦和玉米生长季所面临的气候差异也可能导致根系分泌物对氮循环的影响发生变化。在未来的研究中,可以建立“植物-土壤-微生物”有机串联与协调统一机制,构建土壤氮循环网络体系。
2.3 土壤细菌氮循环功能基因与土壤理化性质的相关性由土壤细菌氮循环功能基因丰度与土壤理化性质的相关性分析可知,与小麦土壤细菌氮循环功能基因关系最为密切的土壤理化性质为SOM、TN和NH4+-N(表 3),玉米土壤细菌氮循环功能基因与土壤TN和TP相关性最为显著(表 4)。在小麦土壤细菌氮异化还原相关基因中,nirB和narL基因与土壤SOM和TN呈显著正相关,narL基因与土壤NH4+-N呈显著负相关;氮同化还原相关基因中,nasA和narB基因与土壤SOM呈显著正相关,nasB基因与土壤TN呈显著正相关;反硝化作用相关基因中,nirK、norB、norC、narG和narH基因均与土壤SOM呈显著正相关,nirK基因还与土壤NH4+-N呈显著负相关,narG和narH基因还与土壤TN呈显著正相关,与土壤NH4+-N呈显著负相关;硝化作用中pmoC-amoC基因与土壤TN呈显著正相关,与NH4+-N呈显著负相关。在玉米土壤细菌氮异化还原相关基因中,nirB基因与土壤TP呈显著正相关,narL基因与土壤TN和TP呈显著正相关,nrfH基因与土壤pH呈显著负相关;氮同化还原相关基因中,nasA、narB和nasB基因与土壤TN和TP呈显著正相关;反硝化作用相关基因中,nirK基因与土壤TP呈显著正相关,narG和narH基因与土壤TN和TP呈显著正相关;硝化作用相关基因均与土壤TP呈显著正相关。土壤细菌氮循环功能基因丰度与土壤理化性质的相关性表明氮循环过程受多种氮循环途径调控,土壤SOM、TN和TP促进氮循环过程,而NH4+-N对氮循环过程产生负面影响。
|
|
表 3 细菌氮循环功能基因与土壤理化性质的相关系数(小麦土壤) Table 3 Correlation coefficients between bacterial nitrogen cycling function genes and soil physical and chemical properties(Wheat soil) |
|
|
表 4 细菌氮循环功能基因与土壤理化性质的相关系数(玉米土壤) Table 4 Correlation coefficients between bacterial nitrogen cycling function genes and soil physical and chemical properties(Maize soil) |
土壤有机质和氮含量对调节土壤氮循环发挥着重要作用[38]。研究表明,土壤有机碳是影响反硝化作用的重要因素[39],土壤有机质提升显著提高了反硝化过程还原酶功能基因nar、nir和nor的丰度[40]。郭安宁等[41]的研究发现土壤硝化作用与NH4+-N含量呈显著负相关,廖李容等[42]的研究发现,narG基因丰度与NH4+-N含量呈显著负相关,这与本研究小麦季的结果相一致。土壤C、N、P等养分供应及其理化指标可以通过改变氮循环功能基因的丰度来影响土壤氮循环过程[23]。小麦和玉米土壤细菌所表现的相关性差异表明影响氮循环功能基因丰度的理化性质并非唯一且不固定,季节动态性变化和施肥方式不同可能会造成时间变异性和环境差异性。轮作体系下不同作物类型会导致土壤微环境的改变,进而影响土壤氮循环功能微生物群落[38, 43]。小麦的须根在其生长阶段可以释放丰富的分泌物,为功能微生物提供内源性碳源[44],推测玉米的须根同样可以通过释放分泌物来为氮循环微生物提供内源性碳源。
3 结论(1)小麦和玉米土壤细菌具有功能上的多样性,有机-无机肥配施在小麦土壤细菌发挥的代谢作用中更为强烈。
(2)小麦和玉米土壤细菌的氮异化还原和氮同化还原潜力最高,反硝化潜力和固氮潜力次之,硝化潜力最弱。其中,氮异化还原过程中nirD和nirB基因,氮同化还原过程中nasA基因,反硝化过程中nirK、narH和narG基因相对丰富。
(3)土壤细菌氮循环功能基因受轮作体系影响,SOM和TN促进小麦土壤氮循环过程,而NH4+-N抑制氮循环过程;TN和TP在玉米土壤细菌氮循环过程中发挥积极作用。
| [1] |
李明姝, 王瑶瑶, 郝毅, 等. 华北小麦玉米轮作体系下土壤重金属污染研究进展[J]. 山东农业科学, 2018, 50(12): 144-151. LI Mingshu, WANG Yao-yao, HAO Yi, et al. Research progress of soil heavy metal pollution under wheat-maize rotation system in the North China[J]. Shandong Agricultural Sciences, 2018, 50(12): 144-151. |
| [2] |
Xiao D P, Tao F L. Contributions of cultivars, management and climate change to winter wheat yield in the North China Plain in the past three decades[J]. European Journal of Agronomy, 2014, 52: 112-122. DOI:10.1016/j.eja.2013.09.020 |
| [3] |
王玉英, 李晓欣, 董文旭, 等. 华北平原农田温室气体排放与减排综述[J]. 中国生态农业学报, 2018, 26(2): 167-174. WANG Yu-ying, LI Xiao-xin, DONG Wen-xu, et al. Review on greenhouse gas emission and reduction in wheat-maize double cropping system in the North China Plain[J]. Chinese Journal of Eco-Agriculture, 2018, 26(2): 167-174. |
| [4] |
夏旭, 李银坤, 陈敏鹏, 等. 碳氮水添加对华北小麦-玉米双季轮作系统碳平衡的影响[J]. 西北大学学报(自然科学版), 2020, 50(2): 250-260. XIA Xu, LI Yin-kun, CHEN Min-peng, et al. Effects of carbon, nitrogen and water addition on carbon balance of wheat-maize double rotation systemin North China Plain[J]. Journal of Northwest University (Natural Science Edition), 2020, 50(2): 250-260. |
| [5] |
Ikoyi I, Egeter B, Chaves C, et al. Responses of soil microbiota and nematodes to application of organic and inorganic fertilizers in grassland columns[J]. Biology and Fertility of Soils, 2020, 56: 647-662. DOI:10.1007/s00374-020-01440-5 |
| [6] |
Ikoyi I, Fowler A, Schmalenberger A. One-time phosphate fertilizer application to grassland columns modifies the soil microbiota and limits its role in ecosystem services[J]. Science of the Total Environment, 2018, 630: 849-858. DOI:10.1016/j.scitotenv.2018.02.263 |
| [7] |
Hu Y J, Xia Y H, Sun Q, et al. Effects of long-term fertilization on phoD-harboring bacterial community in Karst soils[J]. Science of the Total Environment, 2018, 628/629: 53-63. DOI:10.1016/j.scitotenv.2018.01.314 |
| [8] |
叶文培, 谢小立, 王凯荣, 等. 不同时期秸秆还田对水稻生长发育及产量的影响[J]. 中国水稻科学, 2008, 22(1): 65-70. YE Wen-pei, XIE Xiao-li, WANG Kai-rong, et al. Effects of rice straw manuring in different periods on growth and yield of rice[J]. Chinese Journal of Rice Science, 2008, 22(1): 65-70. DOI:10.3321/j.issn:1001-7216.2008.01.011 |
| [9] |
Lori M, Symnaczik S, Mäder P, et al. Organic farming enhances soil microbial abundance and activity:A meta-analysis and meta-regression[J]. PLoS One, 2017, 12(7): e180442. |
| [10] |
Cai F, Pang G, Li R X, et al. Bioorganic fertilizer maintains a more stable soil microbiome than chemical fertilizer for monocropping[J]. Biology and Fertility of Soils, 2017, 53(8): 861-872. DOI:10.1007/s00374-017-1216-y |
| [11] |
Yang Q L, Zheng F X, Jia X C, et al. The combined application of organic and inorganic fertilizers increases soil organic matter and improves soil microenvironment in wheat-maize field[J]. Journal of Soils and Sediments, 2020, 20(5): 2395-2404. DOI:10.1007/s11368-020-02606-2 |
| [12] |
Zhao J, Ni T, Li J, et al. Effects of organic-inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice-wheat cropping system[J]. Applied Soil Ecology, 2016, 99: 1-12. DOI:10.1016/j.apsoil.2015.11.006 |
| [13] |
Zhang M H, Sun D Y, Niu Z R, et al. Effects of combined organic/inorganic fertilizer application on growth, photosynthetic characteristics, yield and fruit quality of Actinidia chinesis cv 'Hongyang'[J]. Global Ecology and Conservation, 2020, 22: e00997. DOI:10.1016/j.gecco.2020.e00997 |
| [14] |
Diacono M, Montemurro F. Long-term effects of organic amendments on soil fertility. A review[J]. Agronomy for Sustainable Development, 2010, 30(2): 401-422. DOI:10.1051/agro/2009040 |
| [15] |
Marschner P, Kandeler E, Marschner B. Structure and function of the soil microbial community in a long-term fertilizer experiment[J]. Soil Biology and Biochemistry, 2003, 35(3): 453-461. DOI:10.1016/S0038-0717(02)00297-3 |
| [16] |
Martínez-García L B, Korthals G, Brussaard L, et al. Organic management and cover crop species steer soil microbial community structure and functionality along with soil organic matter properties[J]. Agriculture, Ecosystems and Environment, 2018, 263: 7-17. DOI:10.1016/j.agee.2018.04.018 |
| [17] |
Kennedy N M, Gleeson D E, Connolly J, et al. Seasonal and management influences on bacterial community structure in an upland grassland soil[J]. FEMS Microbiology Ecology, 2005, 53(3): 329-337. DOI:10.1016/j.femsec.2005.01.013 |
| [18] |
Kennedy N, Brodie E, Connolly J, et al. Seasonal influences on fungal community structure in unimproved and improved upland grassland soils[J]. Canadian Journal Microbiology, 2006, 52(7): 689-694. DOI:10.1139/w06-015 |
| [19] |
Hannula S E, de Boer W, van Veen J, et al. A 3-year study reveals that plant growth stage, season and field site affect soil fungal communities while cultivar and GM-trait have minor effects[J]. PLoS One, 2012, 7(4): e33819. DOI:10.1371/journal.pone.0033819 |
| [20] |
Zhang Z Y, Zhang X K, Mahamood M, et al. Effect of long-term combined application of organic and inorganic fertilizers on soil nematode communities within aggregates[J]. Scientific Reports, 2016, 6(1): 681-693. |
| [21] |
Bei S K, Zhang Y L, Li T T, et al. Response of the soil microbial community to different fertilizer inputs in a wheat-maize rotation on a calcareous soil[J]. Agriculture, Ecosystems and Environment, 2018, 260: 58-69. DOI:10.1016/j.agee.2018.03.014 |
| [22] |
Zhao J, Ni T, Li Y, et al. Responses of bacterial communities in arable soils in a rice-wheat cropping system to different fertilizer regimes and sampling times[J]. PLoS One, 2014, 9(1): e85301. DOI:10.1371/journal.pone.0085301 |
| [23] |
任灵玲.长期施肥棕壤中氮代谢功能基因的变化特征[D].沈阳: 沈阳农业大学, 2019. REN Ling-ling. Characteristics of nitrogencycling-related functional genes under long-term fertilization in brown earth[D]. Shenyang: Shenyang Agricultural University, 2019. |
| [24] |
Merloti L F, Mendes L W, Pedrinho A, et al. Forest-to-agriculture conversion in Amazon drives soil microbial communities and N-cycle[J]. Soil Biology and Biochemistry, 2019, 137: 107567. DOI:10.1016/j.soilbio.2019.107567 |
| [25] |
王梅, 王智慧, 石孝均, 等. 长期不同施肥量对全程氨氧化细菌丰度的影响[J]. 环境科学, 2018, 39(10): 4727-4734. WANG Mei, WANG Zhi-hui, SHI Xiao-jun, et al. Long-term fertilization effects on the abundance of complete ammonia oxidizing bacteria(Comammox Nitrospira) in a neutral paddy soil[J]. Environmental Science, 2018, 39(10): 4727-4734. |
| [26] |
Gilliam F S, Walter C A, Adams M B, et al. Nitrogen(N)dynamics in the mineral soil of a central appalachian hardwood forest during a quarter century of whole-watershed N additions[J]. Ecosystems, 2018, 21: 1489-1504. DOI:10.1007/s10021-018-0234-4 |
| [27] |
Carvalho J L N, Raucci G S, Frazão L A, et al. Crop-pasture rotation:a strategy to reduce soil greenhouse gas emissions in the Brazilian Cerrado[J]. Agriculture, Ecosystems and Environment, 2014, 183: 167-175. DOI:10.1016/j.agee.2013.11.014 |
| [28] |
鲍士旦. 土壤农化分析[M]. 北京: 中国农业出版社, 2000. BAO Shi-dan. Soil agrochemical analysis[M]. Beijing: China Agriculture Press, 2000. |
| [29] |
Wang Q, Wang C, Yu W W, et al. Effects of nitrogen and phosphorus inputs on soil bacterial abundance, diversity, and community composition in Chinese fir plantations[J]. Frontiers in Microbiology, 2018, 9: 1543. DOI:10.3389/fmicb.2018.01543 |
| [30] |
吴宪, 王蕊, 胡菏, 等. 潮土细菌及真菌群落对化肥减量配施有机肥和秸秆的响应[J]. 环境科学, 2020, 41(10): 4669-4681. WU Xian, WANG Rui, HU He, et al. Response of bacterial and fungal communities to chemical fertilizer reduction combined with organic fertilizer and straw in fluvo-aquic soil[J]. Environmental Science, 2020, 41(10): 4669-4681. |
| [31] |
Wang J C, Song Y, Ma T F, et al. Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil[J]. Applied Soil Ecology, 2017, 112: 42-50. DOI:10.1016/j.apsoil.2017.01.005 |
| [32] |
Chen Z, Hou H J, Zheng Y, et al. Influence of fertilisation regimes on a nosZ-containing denitrifying community in a rice paddy soil[J]. Journal of the Science of Food and Agriculture, 2012, 92(5): 1064-1072. DOI:10.1002/jsfa.4533 |
| [33] |
Finzi A C, Abramoff R Z, Spiller K S, et al. Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles[J]. Global Change Biology, 2015, 21(5): 2082-2094. DOI:10.1111/gcb.12816 |
| [34] |
Coskun D, Britto D T, Shi W M, et al. How plant root exudates shape the nitrogen cycle[J]. Trends in Plant Science, 2017, 22(8): 661-673. DOI:10.1016/j.tplants.2017.05.004 |
| [35] |
O'Sullivan C A, Fillery I R P, Roper M M, et al. Identification of several wheat landraces with biological nitrification inhibition capacity[J]. Plant Soil, 2016, 404(1/2): 61-74. |
| [36] |
Ladha J K, Tirol-Padre A, Reddy C K, et al. Global nitrogen budgets in cereals:A 50-year assessment for maize, rice, and wheat production systems[J]. Scientific Reports, 2016, 6: 19355. DOI:10.1038/srep19355 |
| [37] |
Yin H J, Li Y F, Xiao J, et al. Enhanced root exudation stimulates soil nitrogen transformations in a subalpine coniferous forest under experimental warming[J]. Global Change Biology, 2013, 19(7): 2158-2167. DOI:10.1111/gcb.12161 |
| [38] |
杨丹, 余旋, 刘旭, 等. 栽培模式对沙棘人工林土壤微生物群落结构和参与氮循环功能基因的影响[J]. 应用生态学报, 2015, 26(12): 3634-3640. YANG Dan, YU Xuan, LIU Xu, et al. Effect of afforestation modes on soil microbial community and nitrogen functional genes in Hippophae rhamnoides plantation[J]. Chinese Journal of Applied Ecology, 2015, 26(12): 3634-3640. |
| [39] |
Levy-Booth D J, Prescott C E, Grayston S J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems[J]. Soil Biology and Biochemistry, 2014, 75: 11-25. DOI:10.1016/j.soilbio.2014.03.021 |
| [40] |
储成, 吴赵越, 黄欠如, 等. 有机质提升对酸性红壤氮循环功能基因及功能微生物的影响[J]. 环境科学, 2020, 41(5): 2468-2475. CHU Cheng, WU Zhao-yue, HUANG Qian-ru, et al. Effect of organic matter promotion on nitrogen-cycling genes and functional microorganisms in acidic red soils[J]. Environmental Science, 2020, 41(5): 2468-2475. |
| [41] |
郭安宁, 段桂兰, 赵中秋, 等. 施加碳酸钙对酸性土壤微生物氮循环的影响[J]. 环境科学, 2017, 38(8): 3483-3488. GUO An-ning, DUAN Gui-lan, ZHAO Zhong-qiu, et al. Effect of organic matter promotion on nitrogen-cycling genes and functional microorganisms in acidic red soils[J]. Environmental Science, 2017, 38(8): 3483-3488. |
| [42] |
廖李容, 王杰, 张超, 等. 禁牧对半干旱草地土壤氮循环功能基因丰度和氮储量的影响[J]. 应用生态学报, 2019, 30(10): 3473-3481. LIAO Li-rong, WANG Jie, ZHANG Chao, et al. Effects of grazing exclusion on the abundance of functional genes involved in soil nitrogen cycling and nitrogen storage in semiarid grassland[J]. Chinese Journal of Applied Ecology, 2019, 30(10): 3473-3481. |
| [43] |
张晶, 张惠文, 李新宇, 等. 土壤微生物生态过程与微生物功能基因多样性[J]. 应用生态学报, 2006, 17(6): 1129-1132. ZHANG Jing, ZHANG Hui-wen, LI Xin-yu, et al. Soil microbial ecological process and microbial functional gene diversity[J]. Chinese Journal of Applied Ecology, 2006, 17(6): 1129-1132. DOI:10.3321/j.issn:1001-9332.2006.06.034 |
| [44] |
倪雷.施用有机肥料对水旱轮作系统小麦土壤微生物区系的影响[D].南京: 南京农业大学, 2018. NI Lei. The effects of application of organic fertilizer on the flora of wheat soil bacteria in wheat-rice rotation system[D]. Nanjing: Nanjing Agricultural University, 2018. |
 2021, Vol. 40
2021, Vol. 40