迄今为止,抗生素仍是人类对抗细菌性感染的重要手段。由于其不当使用等问题,环境中的抗生素残留及相关抗生素耐药基因(Antibiotic resistance genes,ARGs)已经成为威胁公共健康安全的环境污染物,世界上每年有多达70万人因耐药性问题死亡[1]。抗生素耐药性问题是世界各国面临的共同挑战,我国的耐药性问题也不容忽视。我国是目前世界上最大的抗生素原料药生产国和抗生素产品消费国,供应全球90% 的抗生素原料药市场[2],年产量超过100万t[3],年消费量约为世界总量的12.5%[4],抗生素生产过程中的制药废弃物[5]、人和动物给药后的粪尿[6-7]以及食物垃圾[8]和污泥[9]等都已经成为事实上的抗生素及ARGs储藏库。堆肥是一种常用的有机固废处理技术,在抗生素降解及其ARGs消减方面也有重要作用[7, 10],充分了解堆肥过程中抗生素及其ARGs消减的规律、影响因素及优化技术,不仅对我国有机固废利用具有重要意义,也为最大限度减少抗生素及ARGs扩散风险提供重要参考。
1 研究现状分析方法在Web of Science数据库中以TS=“compost”AND “antibiotic”以及TI=“compost”and(TS=“antibiotic” OR“antimicrobial”OR“pharmaceutical”OR“drug”OR “substance”OR“ARG”OR“ARB”OR“resistance”OR “security”OR“emerging contaminants”),分两次筛选文献(截至2021年6月25日),筛选条件为:(1)堆肥最高温度达到50 ℃及以上;(2)有完整的升温和降温过程;(3)至少报告了一种或一类或总抗生素/ARGs在堆肥前后的浓度或丰度变化;(4)报告了堆肥原料和堆肥方式。共筛选到132篇文献。
抗生素及ARGs在堆肥过程中的消减规律研究已成为近年来(特别是最近5 a)的热点问题(图 1),通过堆肥技术降低ARGs的传播风险逐渐引发关注。

|
图 1 相关文献发表年度变化 Figure 1 The number of relevant literatures in recent years |
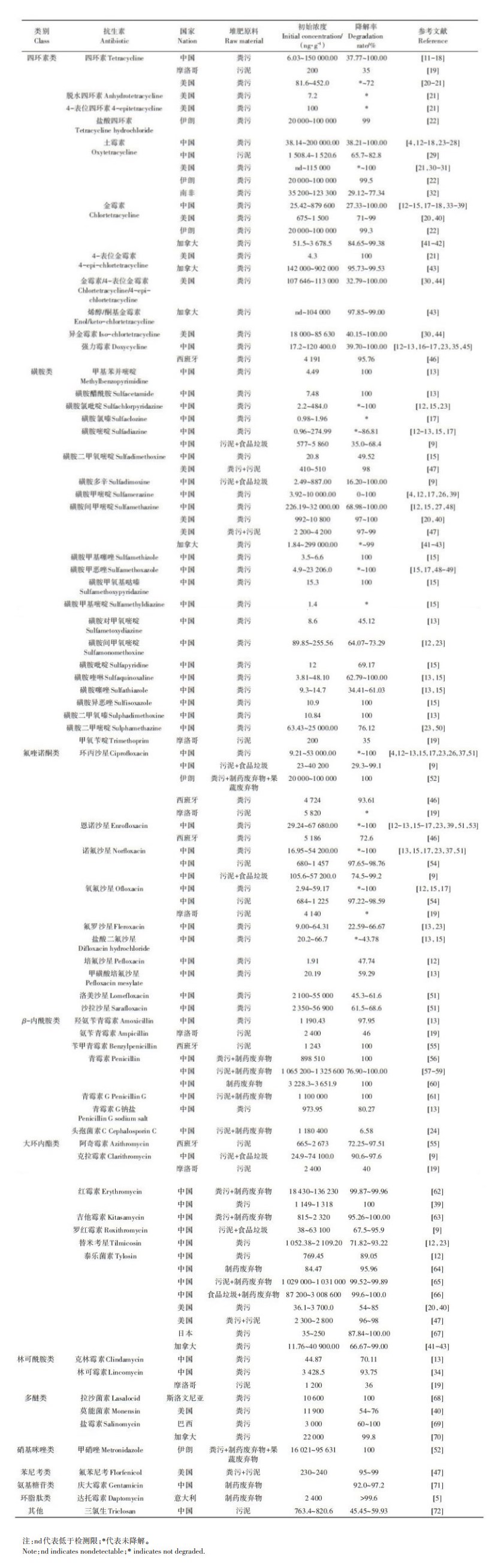
我国既是抗生素生产大国[2],也是抗生素产品消费大国[4],面临的ARGs污染风险尤为突出,对于堆肥过程中抗生素及ARGs消减规律关注度更高,其次是美国和加拿大,其他国家在这一领域的聚焦相对较少(图 2)。相关研究在我国分布区域并不均衡,以北京(19.6%)和陕西(18.7%)最为集中,其次为黑龙江(13.1%),其他地区相对较少。
|
图 2 相关文献国际分布情况 Figure 2 Distribution of nations and areas of relevant studies |
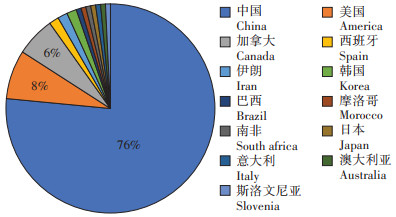
粪污、污泥和制药废弃物是抗生素及ARGs在堆肥中消减规律研究中涉及的主要堆肥原料,也是主要的抗生素及ARGs污染风险来源,其中粪污是最重要的研究原料,76.5%的相关研究完全或部分以粪污为主要堆肥原料(图 3),17.4% 的相关研究完全或部分以污泥为主要堆肥原料,15.2%的相关研究完全或部分以制药废弃物为主要堆肥原料。
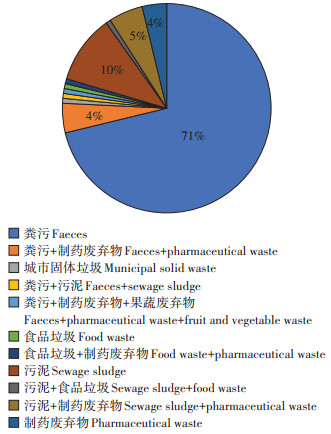
|
图 3 相关文献堆肥主要原料 Figure 3 Distribution of main raw materials of relevant literatures |
堆肥能够有效降解多种抗生素(表 1)。其中,磺胺类[20]、喹诺酮类[23]、四环素类[33]、大环内酯类[63]、β-内酰胺类[57]抗生素甚至可以降至检测限以下,甲硝唑[52]、盐霉素[70]、三氯卡班[29]、氟苯尼考[47]、拉沙菌素[68]、达托霉素[5]和林可酰胺[20]等抗生素也能得到有效降解。
|
|
表 1 抗生素在堆肥中消减情况 Table 1 The degradation of antibiotics during composting |
有些堆肥试验中抗生素降解率较低,如火鸡粪便堆肥中,磺胺类抗生素未能降解[40],污泥堆肥[19]和商业化畜禽粪污堆肥[34]中,氟喹诺酮类抗生素几乎没有变化,四环素类抗生素在阉公猪粪便堆肥中降解率仅为27.33%[35],牛粪堆肥中泰乐菌素基本没有降解[20],头孢菌素C菌渣和污泥共堆肥中头孢菌素C的降解率仅为6.58%[24]。
总体来说,大部分抗生素均能在适当的堆肥处理中得到有效降解,但相同堆肥处理中不同类别的抗生素以及同类抗生素在不同堆肥处理中的降解率差别很大,特别是部分试验处理中出现抗生素降解率低甚至基本没有降解的情况,这种差异可能是受到堆肥原料理化性质、抗生素浓度差异、堆体温度变化等因素影响。
2.2 堆肥过程中ARGs及移动基因元件(Mobile genetic elements,MGEs)消减情况堆肥可以大幅降低沼渣[11]和城市固体垃圾[53]以及大部分畜禽粪污[6, 46]等有机固废中的ARGs,但部分ARGs,特别是sul1在畜禽废弃物[73]和城市污泥[74]等多种有机固废堆肥中都很难清除,且堆肥腐熟期往往发生反弹[75],导致消减效率较低甚至在堆肥后不降反升等问题[76]。
ARGs大部分位于MGEs中[77],其丰度变化受到MGEs的强烈影响[78],intl1与ARGs丰度变化正相关[10]。在城市固体垃圾[53]、畜禽粪污[6, 46, 79]堆肥过程中,intl1的丰度均出现不同程度上升或始终保持在较高水平。堆肥原料及堆肥处理等因素对intl1的消减都有影响,例如,鸡粪堆肥不能有效降低intl1丰度[46],但在鸡粪与中药渣共堆肥过程中intl1相对丰度显著下降[80]。
sul1和intl1被普遍认为是人源耐药性和降解效果的重要监测指标[6],且存在共轭传播[12],在各类堆肥中广泛存在,应予以重点关注。
3 堆肥过程中抗生素及ARGs消减的影响因素 3.1 堆肥原料堆肥原料中的抗生素甚至制药废弃物[24, 60]中残留的超高浓度抗生素往往并不影响堆肥腐熟,但会影响堆体菌群结构并导致ARGs丰度上升。如:青霉素制药废弃物堆肥过程中各理化指标均能达到腐熟标准,青霉素浓度也降低至检测限以下[60],但堆肥后ARGs丰度明显上升[61]。加热预处理能够有效降低β-内酰胺类[81]、四环素类[82]、大环内酯类[83]等抗生素制药废弃物堆肥过程中的ARGs丰度[66]。
堆肥填充料对抗生素[58]及ARGs[84]消减的作用较小。例如,木屑在堆肥中只起到改善堆体通透性的作用,基本不参与微生物作用[60],堆肥填充料是否来自转基因作物对ARGs消减也没有明显影响[85]。但需要注意其本身存在的ARGs传播风险。如:将含有耐药菌(Antibiotic resistance bacterial,ARB)的玉米秸秆用作堆肥填充料,最终堆肥成品中ARB检出率甚至高于静态堆放处理[86]。
3.2 菌群结构微生物菌群结构是影响堆肥中抗生素及其ARGs消减的主要因素[67, 76]。厚壁菌门、变形菌门、拟杆菌门、放线菌门是最重要的ARGs潜在宿主菌门[12-13, 87],此外还有部分古菌(如嗜盐菌属[88])。在具体堆肥过程中,潜在宿主菌除受原料及堆肥时期的影响外,在堆体不同深度的分布也有差异[89]。
3.3 理化性质堆肥中适当增加碳(C)投入有利于ARGs消减[76],C/N[90]、可溶性S[53]、pH[53]对ARGs宿主菌都具有重要影响。pH还会影响抗生素降解过程[52],如青霉素在碱性堆肥中更易降解[91]。
堆肥产生的高温能够明显促进抗生素[14, 47]及ARGs消减[52],但温度并不是影响抗生素降解的直接因素,而是通过改变微生物菌群结构影响抗生素降解[67]。超高温堆肥去除ARGs的机理与传统堆肥不同,能够去除几乎全部的MGEs,从而限制ARGs的水平转移,相比于传统堆肥更利于ARGs消减[77]。
总体来说,堆体理化性质,如C、N、S等元素含量及pH、温度等都会影响堆肥过程中抗生素及ARGs消减,但这些因素的影响往往是间接的,通过改变堆肥过程中的微生物菌群发挥作用。
3.4 重金属高浓度Cu含量会抑制磺胺类[48]、四环素类[36]抗生素降解,对于泰乐菌素[65]的降解影响较小。高浓度Zn则会导致堆体温度峰值降低和滞后[50]。
生物有效态Cu、Zn与ARGs丰度存在共轭性[76, 92],可能是因为ARGs和重金属耐药基因(Heavy metal resistance genes,MRGs)有共同的宿主微生物[93],高浓度的生物有效态Cu、Zn会对微生物的抗生素耐药性产生共同的选择压力[94],引起部分ARGs丰度上升[42, 58],重金属钝化则利于ARGs消减[95]。
3.5 其他有关抗生素在堆肥过程中消减的研究中,往往在堆肥材料(如畜禽粪污等)中人为添加抗生素以形成处理之间更高的浓度差,但人工添加至畜禽粪污中的抗生素与畜禽饲喂过程中给药后残留在粪污中的抗生素在堆肥过程中的降解规律及其对ARGs的影响并不完全一致。人工添加的磺胺类和大环内酯类抗生素在堆肥过程中降解更快,四环素类抗生素则在动物给药后排泄的粪污堆肥中降解更快[41]。另外,畜禽给药方式使ARGs宿主菌群受到选择压力时间更长,ARGs也更不易消减[42]。总体来讲,人工添加抗生素的试验处理方式并不能准确反映生产条件下畜禽粪污堆肥过程,还应当进一步予以印证。
此外,堆肥时间[52]、含水率及翻堆频次[34]、堆肥标准化程度[15]都会对抗生素降解及ARGs消减产生显著影响。堆体不同部位的ARGs消减率也有所不同,堆体中心优于顶层,底层的消减率最低[7],这可能是堆体不同部位的透气性、保温性等方面差异导致的。堆肥厂的空气环境同样显著影响腐熟堆肥中的ARGs丰度变化[96],这可能与堆肥过程中抗生素挥发导致空气环境中存在一定的抗生素及ARGs有关[54]。
4 堆肥优化工艺对抗生素及其ARGs消减的影响传统堆肥在抗生素降解(特别是其ARGs消减)方面仍然存在诸多局限性,在传统堆肥基础上进行优化和强化很有必要。常见的堆肥优化工艺见表 2。
|
|
表 2 堆肥优化工艺 Table 2 Enhancing methods of composting |
合理优化的堆肥工艺可以有效提高畜禽粪污处理效率,就抗生素降解效果来看,发酵罐堆肥的抗生素消减效果优于控制通风的条垛堆肥和静态堆制堆肥,可以在一周内达到商业堆肥标准[115]。持续通风优于间断通风和不通风[54],负压通气方式比正压通气方式更利于ARGs和intl1的消除[117],这可能与负压通气方式更利于减少引入空气中的抗生素及ARGs有关。半透膜覆膜堆肥也能够有效阻遏空气环境对腐熟堆肥中ARGs丰度反弹的促进作用,提升ARGs消减效果[96]。
4.2 添加外源微生物在堆肥中加入外源微生物的主要方式有添加堆肥成品[73]、添加配制菌剂等。引入外源菌剂能够改变堆体微生物群落结构,提高堆肥温度峰值,延长堆肥高温期[7, 73]。研究证实,适当添加外源菌剂对于抗生素及ARGs的消减具有明显促进作用[97, 16],在堆肥过程中采用的菌种有黄孢原毛平革菌、地衣芽孢杆菌、黑曲霉[16]、土曲霉[71]、枯草芽孢杆菌[98]、木质素降解菌[99]等,复合菌剂施用效果往往优于单一菌剂[45]。
4.3 堆肥添加剂堆肥添加剂对于ARGs消减的影响已经成为目前堆肥优化技术研究热点之一,前人对生物炭和沸石[10]、泥炭[95]、虾壳粉[109]、玉米穗轴[76]、褐煤[107]、竹醋[75]、纳米零价铁[114]、铁基材料和溶磷剂[100]、生物表面活性剂(鼠李糖脂)和化学表面活性剂(吐温80)[113]、过磷酸钙[105]、砖粒[76]、红泥[108]、3,4 -二甲基吡唑磷酸盐(DMPP)等不同材料对堆肥过程中ARGs消减的影响进行了研究。除砖粒、红泥和DMPP外,大部分材料对ARGs消减有一定积极意义。
生物炭类添加剂虽然广受关注,但其对于ARGs消减的作用尚不明确。大部分研究认为添加生物炭(如竹炭[37)] 对于堆肥过程中ARGs的消减有促进作用[75, 101],但有研究发现,稻草生物炭会促进部分畜禽粪污[92, 102]堆肥中ARGs丰度增加,蘑菇生物炭能降低鸡粪[92]和猪粪[102]堆肥中ARGs丰度,但会促进鸭粪[102]堆肥中ARGs丰度增加。也有研究认为,生物炭添加比例是影响堆肥过程ARGs消减的主要因素,而非生物炭的种类[103]。
不可逆吸附是抗生素在堆肥过程中消减的重要途径之一[54],在堆体中添加适量的大孔吸附树脂[111]、沸石[106]等多孔结构材料,有利于增强堆体物理吸附能力,促进ARGs和MGEs的消减。
5 展望 5.1 筛选堆肥复合菌剂目前,已有部分研究对堆肥菌剂进行了探索,并在促进抗生素及其ARGs消减方面取得一定成果,但相关菌种资源挖掘还比较有限,今后的研究中应当进一步筛选相关高效功能菌,明确不同菌种之间的协同及拮抗作用,探究能够同时有效去除多种抗生素及ARGs的复合菌剂。
5.2 重视规模堆肥研究目前,抗生素及其ARGs在堆肥过程中的消减规律研究以实验室水平及中试水平堆肥试验为主,多以人工添加抗生素的方式形成处理间的浓度梯度,但抗生素及ARGs在工业水平堆肥中的消减规律与模拟堆肥及小规模堆肥试验中并不完全一致,往往受到更多因素影响,人工添加到粪污等堆肥材料中的抗生素与动物给药后残留在粪污中的抗生素消减规律及对ARGs的诱导效应也有差异,今后的研究应在实验室及中试试验基础上,更加重视在工业水平堆肥中对相关规律的验证,提高相关成果对于生产实践的指导意义。
5.3 优化堆肥技术目前,堆肥对于病原微生物的杀灭作用及为作物提供稳定养分等方面的作用已经得到普遍认同,但在降低耐药风险方面的研究尚不成熟,也未能形成完整的技术体系。已经有研究分析了理化指标、微生物菌群结构、重金属、堆肥管理、空气环境及添加剂等诸多因素对堆肥过程中抗生素及ARGs的影响,今后的研究中还应加强对各类影响因素的整合分析,逐步形成针对不同原料的堆肥技术体系。
5.4 深挖分子机理目前,关于抗生素及ARGs在堆肥过程中消减规律的研究大多是对其在具体试验处理中的消减特征进行表述,并通过相关性数据分析理化指标、微生物群落结构、重金属等因素对抗生素浓度及ARGs丰度变化的贡献度,但不同堆肥试验中抗生素及ARGs消减效果往往差别较大,其具体分子机理尚不明确,今后的研究中还应该进一步深挖相关分子机理并逐渐完善理论基础,提高相关研究的针对性。
| [1] |
刘昌孝. 全球关注: 重视抗生素发展与耐药风险的对策[J]. 中国抗生素杂志, 2019, 44(1): 1-8. LIU C X. Global concern: Strategies for antibiotic development and risk of resistance[J]. Chinese Journal of Antibiotics, 2019, 44(1): 1-8. DOI:10.3969/j.issn.1001-8689.2019.01.001 |
| [2] |
肖祖飞. 制药污泥的堆肥化对抗生素降解及ARGs转移的影响机制研究[D]. 苏州: 苏州科技大学, 2019: 1-2. XIAO Z F. Mechanisms on effects of pharmaceutical sewage sludge composting on antibiotic degradation and ARGs transfer[D]. Suzhou: Suzhou University of Science and Technology, 2019: 1-2. |
| [3] |
刘园园. 我国抗生素菌渣处置技术现状[J]. 中国环保产业, 2017(8): 66-68. LIU Y Y. Technical status of antibiotic bacterium dregs disposal[J]. China Environmental Protection Industry, 2017(8): 66-68. DOI:10.3969/j.issn.1006-5377.2017.08.012 |
| [4] |
CHENG D, LIU Y, SHEHATA E, et al. In-feed antibiotic use changed the behaviors of oxytetracycline, sulfamerazine, and ciprofloxacin and related antibiotic resistance genes during swine manure composting[J]. Journal of Hazardous Materials, 2021, 402: 123710. DOI:10.1016/j.jhazmat.2020.123710 |
| [5] |
MIRKO C, CLAUDIA Z, MARIA C M, et al. Recovery of energy and plant nutrients from a pharmaceutical organic waste derived from a fermentative biomass: Integration of anaerobic digestion and composting[J]. Journal of Environmental Chemical Engineering, 2017, 5(3): 3051-3057. DOI:10.1016/j.jece.2017.06.003 |
| [6] |
KEENUM I, WILLIAMS R K, RAY P, et al. Combined effects of composting and antibiotic administration on cattle manure-borne antibiotic resistance genes[J]. Microbiome, 2021, 9: 81. DOI:10.1186/s40168-021-01006-z |
| [7] |
LI K, CAO R, MO S, et al. Swine manure composting with compound microbial inoculants: removal of antibiotic resistance genes and their associations with microbial community[J]. Frontiers in Microbiology, 2020, 3: 592592. |
| [8] |
LIAO H, FRIMAN V P, GEISEN S, et al. Horizontal gene transfer and shifts in linked bacterial community composition are associated with maintenance of antibiotic resistance genes during food waste composting[J]. Science of the Total Environment, 2019, 660: 841-850. DOI:10.1016/j.scitotenv.2018.12.353 |
| [9] |
CHEN Z, LI Y, YE C, et al. Fate of antibiotics and antibiotic resistance genes during aerobic co-composting of food waste with sewage sludge[J]. Science of the Total Environment, 2021, 784: 146950. DOI:10.1016/j.scitotenv.2021.146950 |
| [10] |
ZHOU G, QIU X, WU X, et al. Horizontal gene transfer is a key determinant of antibiotic resistance genes profiles during chicken manure composting with the addition of biochar and zeolite[J]. Journal of Hazardous Materials, 2021, 408: 124883. DOI:10.1016/j.jhazmat.2020.124883 |
| [11] |
LIN Z, WU Z L, SUN Z Y, et al. Aerobic post-treatment of anaerobic digested sludge with a focus on organic matter stability and the fate of antibiotic resistance genes[J]. Journal of Cleaner Production, 2021, 125798. |
| [12] |
LIN H, SUN W, JIN D, et al. Effect of composting on the conjugative transmission of sulfonamide resistance and sulfonamide-resistant bacterial population[J]. Journal of Cleaner Production, 2021, 285: 125483. DOI:10.1016/j.jclepro.2020.125483 |
| [13] |
WANG G, LI G, CHANG J, et al. Enrichment of antibiotic resistance genes after sheep manure aerobic heap composting[J]. Bioresource Technology, 2021, 323: 124260. |
| [14] |
WU X, WEI Y, ZHENG J, et al. The behavior of tetracyclines and their degradation products during swine manure composting[J]. Bioresource Technology, 2011, 102(10): 5924-5931. DOI:10.1016/j.biortech.2011.03.007 |
| [15] |
LIU H, PU C, YU X, et al. Removal of tetracyclines, sulfonamides, and quinolones by industrial-scale composting and anaerobic digestion prosemicesses[J]. Environmental Science and Pollution Research, 2018, 25(36): 35835-35844. DOI:10.1007/s11356-018-1487-3 |
| [16] |
LIU Y, ZHENG L, CAI Q, et al. Simultaneous reduction of antibiotics and antibiotic resistance genes in pig manure using a composting process with a novel microbial agent[J]. Ecotoxicology and Environmental Safety, 2021, 208: 111724. DOI:10.1016/j.ecoenv.2020.111724 |
| [17] |
WANG C, DONG D, STRONG P J, et al. Microbial phylogeny determines transcriptional response of resistome to dynamic composting processes[J]. Microbiome, 2017, 5: 103. DOI:10.1186/s40168-017-0324-0 |
| [18] |
CHAI R, HUANG L, LI L, et al. Degradation of tetracyclines in pig manure by composting with rice straw[J]. International Journal of Environmental Research and Public Health, 2016, 13(3): 254. DOI:10.3390/ijerph13030254 |
| [19] |
AHMED K, AMINE E, GEORGES M, et al. Fate of antibiotics present in a primary sludge of WWTP during their co-composting with palm wastes[J]. Waste Management, 2019, 84: 13-19. DOI:10.1016/j.wasman.2018.11.009 |
| [20] |
PARTHA R, CHEN C, KATHARINE F K, et al. Fate and effect of antibiotics in beef and dairy manure during static and turned composting[J]. Journal of Environmental Quality, 2017, 46: 45-54. DOI:10.2134/jeq2016.07.0269 |
| [21] |
SCHUELER J, NAAS K, HURST J, et al. Effects of on-farm dairy manure composting on tetracycline content and nutrient composition[J]. Antibiotics (Basel, Switzerland), 2021, 10(4): 10040443. |
| [22] |
NADALI A, KHATEREH S, GHOLAMREZA G, et al. Attenuation of tetracyclines during chicken manure and bagasse co-composting: Degradation, kinetics, and artificial neural network modeling[J]. Journal of Environmental Management, 2019, 231: 1203-1210. DOI:10.1016/j.jenvman.2018.11.003 |
| [23] |
朱为静, 朱凤香, 王卫平, 等. 4种粪便堆肥过程中抗生素的降解特性[J]. 环境科学, 2020, 41(2): 1005-1012. ZHU W J, ZHU F X, WANG W P, et al. Degradation characteristics of antibiotics during composting of four types of feces[J]. Environmental Science, 2020, 41(2): 1005-1012. |
| [24] |
CHEN Z, WANG Y, WEN Q, et al. Feasibility study of recycling cephalosporin C fermentation dregs using co-composting process with activated sludge as co-substrate[J]. Environmental Technology, 2016, 37(17): 2222-2230. DOI:10.1080/09593330.2016.1146340 |
| [25] |
SHEHATA E, LIU Y, FENG Y, et al. Changes in arsenic and copper bioavailability and oxytetracycline degradation during the composting process[J]. Molecules, 2019, 24(23): 4240. DOI:10.3390/molecules24234240 |
| [26] |
CHENG D, FENG Y, LIU Y, et al. Dynamics of oxytetracycline, sulfamerazine, and ciprofloxacin and related antibiotic resistance genes during swine manure composting[J]. Journal of Environmental Management, 2018, 230: 102-109. |
| [27] |
WANG J, BEN W, ZHANG Y, et al. Effects of thermophilic composting on oxytetracycline, sulfamethazine, and their corresponding resistance genes in swine manure[J]. Environmental Science Processes & Impacts, 2015, 17(9): 1654-1660. |
| [28] |
SHEHATA E, CHENG D, MA Q, et al. Microbial community dynamics during composting of animal manures contaminated with arsenic, copper, and oxytetracycline[J]. Journal of Integrative Agriculture, 2021, 20(6): 1649-1659. DOI:10.1016/S2095-3119(20)63290-7 |
| [29] |
ZHENG G, YU B, WANG Y, et al. Fate and biodegradation characteristics of triclocarban in wastewater treatment plants and sewage sludge composting processes and risk assessment after entering the ecological environment[J]. Journal of Hazardous Materials, 2021, 412: 125270. DOI:10.1016/j.jhazmat.2021.125270 |
| [30] |
ARIKAN O, MULBRY W, INGRAM D, et al. Minimally managed composting of beef manure at the pilot scale: Effect of manure pile construction on pile temperature profiles and on the fate of oxytetracycline and chlortetracycline[J]. Bioresource Technology, 2009, 100(19): 4447-4453. DOI:10.1016/j.biortech.2008.12.063 |
| [31] |
ARIKAN O A, SIKORA L J, MULBRY W, et al. Composting rapidly reduces levels of extractable oxytetracycline in manure from therapeutically treated beef calves[J]. Bioresource Technology, 2007, 98(1): 169-176. DOI:10.1016/j.biortech.2005.10.041 |
| [32] |
RAVINDRAN B, MNKENI P N S. Identification and fate of antibiotic residue degradation during composting and vermicomposting of chicken manure[J]. International Journal of Environmental Science and Technology, 2017, 14(2): 263-270. DOI:10.1007/s13762-016-1131-z |
| [33] |
CHEN Z, FU Q, CAO Y, et al. Effects of lime amendment on the organic substances changes, antibiotics removal, and heavy metals speciation transformation during swine manure composting[J]. Chemosphere, 2021, 262: 128342. DOI:10.1016/j.chemosphere.2020.128342 |
| [34] |
ZHANG M, HE L Y, LIU Y S, et al. Fate of veterinary antibiotics during animal manure composting[J]. Science of the Total Environment, 2019, 650: 1363-1370. DOI:10.1016/j.scitotenv.2018.09.147 |
| [35] |
BAO Y, ZHOU Q, GUAN L, et al. Depletion of chlortetracycline during composting of aged and spiked manures[J]. Waste Management, 2009, 29(4): 1416-1423. DOI:10.1016/j.wasman.2008.08.022 |
| [36] |
温沁雪, 曹永森, 陈志强. 猪粪堆肥过程中金霉素去除及重金属形态变化[J]. 环境科学, 2017, 38(10): 4405-4411. WEN Q X, CAO Y S, CHEN Z Q. Removal of chlortetracycline and morphological changes in heavy metals in swine manure using the composting process[J]. Environmental Science, 2017, 38(10): 4405-4411. |
| [37] |
WANG L, CHEN G, GARY O, et al. Enhanced antibiotic removal by the addition of bamboo charcoal during pig manure composting[J]. RSC Advances, 2016, 6(33): 27575-27583. DOI:10.1039/C5RA27493A |
| [38] |
WANG Y, CHEN Z, WEN Q, et al. Variation of heavy metal speciation, antibiotic degradation, and potential horizontal gene transfer during pig manure composting under different chlortetracycline concentration[J]. Environmental Science and Pollution Research International, 2021, 28: 1224-1234. DOI:10.1007/s11356-020-10557-x |
| [39] |
CHEN Z, WANG Y, WEN Q. Effects of chlortetracycline on the fate of multi-antibiotic resistance genes and the microbial community during swine manure composting[J]. Environmental Pollution, 2018, 237: 977-987. DOI:10.1016/j.envpol.2017.11.009 |
| [40] |
HOLLY D, SATISH G, SALLY N. Antibiotic degradation during manure composting[J]. Journal of Environmental Quality, 2008, 37(3): 1245-1253. DOI:10.2134/jeq2007.0399 |
| [41] |
INOKA D A, FRANCIS Z, SRINIVAS S, et al. Dissipation of antimicrobials in feedlot manure compost after oral administration versus fortification after excretion[J]. Journal of Environmental Quality, 2016, 45(2): 503-510. DOI:10.2134/jeq2015.07.0408 |
| [42] |
XU S, AMARAKOON I D, ZAHEER R, et al. Dissipation of antimicrobial resistance genes in compost originating from cattle manure after direct oral administration or post-excretion fortification of antimicrobials[J]. Journal of Environmental Science and Health, 2018, 53(4): 373-384. DOI:10.1080/10934529.2017.1404337 |
| [43] |
CESSNA A J, LARNEY F J, KUCHTA S L, et al. Veterinary antimicrobials in feedlot manure: Dissipation during composting and effects on composting processes[J]. Journal of Environmental Quality, 2011, 40(1): 188-198. DOI:10.2134/jeq2010.0079 |
| [44] |
ARIKAN O A, MULBRY W, Rice C. Management of antibiotic residues from agricultural sources: Use of composting to reduce chlortetracycline residues in beef manure from treated animals[J]. Journal of Hazardous Materials, 2009, 164: 483-489. DOI:10.1016/j.jhazmat.2008.08.019 |
| [45] |
LIANG J, JIN Y, WEN X, et al. Adding a complex microbial agent twice to the composting of laying-hen manure promoted doxycycline degradation with a low risk on spreading tetracycline resistance genes[J]. Environmental Pollution, 2020, 265: 114202. DOI:10.1016/j.envpol.2020.114202 |
| [46] |
FERNANDO E, BEATRIZ A, MARÍA U R, et al. Assessing the benefits of composting poultry manure in reducing antimicrobial residues, pathogenic bacteria, and antimicrobial resistance genes: A field-scale study[J]. Environmental Science and Pollution Research, 2020, 27(22): 27738-27749. DOI:10.1007/s11356-020-09097-1 |
| [47] |
MITCHELL S M, ULLMAN J L, BARY A, et al. Antibiotic degradation during thermophilic composting[J]. Water, Air, & Soil Pollution, 2015, 226(2): 13. |
| [48] |
LIU B, LI Y, ZHANG X, et al. Effects of composting process on the dissipation of extractable sulfonamides in swine manure[J]. Bioresource Technology, 2015, 175: 284-290. DOI:10.1016/j.biortech.2014.10.098 |
| [49] |
SARDAR M F, ZHU C, GENG B, et al. The fate of antibiotic resistance genes in cow manure composting: Shaped by temperature-controlled composting stages[J]. Bioresource Technology, 2021, 320: 124403. DOI:10.1016/j.biortech.2020.124403 |
| [50] |
DUAN M, YANG J, JIE G, et al. Effects of sulphamethazine and zinc on the functional diversity of microbial communities during composting[J]. Environmental Technology, 2016, 37(11): 1357-1368. DOI:10.1080/09593330.2015.1115131 |
| [51] |
YANG B, MENG L, XUE N. Removal of five fluoroquinolone antibiotics during broiler manure composting[J]. Environmental Technology, 2018, 39(3): 373-381. DOI:10.1080/09593330.2017.1301568 |
| [52] |
JONIDI J, AHMAD F, MAHDI G, et al. The efficiency of removing metronidazole and ciprofloxacin antibiotics as pharmaceutical wastes during the process of composting[J]. International Journal of Environmental Analytical Chemistry, 2020, 1781838. |
| [53] |
TANG Z, XI B, HUANG C, et al. Mobile genetic elements in potential host microorganisms are the key hindrance for the removal of antibiotic resistance genes in industrial-scale composting with municipal solid waste[J]. Bioresource Technology, 2020, 301: 122723. DOI:10.1016/j.biortech.2019.122723 |
| [54] |
ZHANG J, BAO Y, JIANG Y, et al. Removal and dissipation pathway of typical fluoroquinolones in sewage sludge during aerobic composting[J]. Waste Management, 2019, 95: 450-457. DOI:10.1016/j.wasman.2019.06.036 |
| [55] |
MARÍA I, MIGUEL G, RAFAEL B, et al. Analysis of pharmaceutical biodegradation of WWTP sludge using composting and identification of certain microorganisms involved in the process[J]. Science of the Total Environment, 2018, 640/641: 840-848. |
| [56] |
ZHANG Z, ZHAO J, YU C, et al. Evaluation of aerobic co-composting of penicillin fermentation fungi residue with pig manure on penicillin degradation, microbial population dynamics and composting maturity[J]. Bioresource Technology, 2015, 198: 403-409. DOI:10.1016/j.biortech.2015.09.005 |
| [57] |
ZHANG S, CHEN Z, WEN Q, et al. Assessment of maturity during co-composting of penicillin mycelial dreg via fluorescence excitationemission matrix spectra: Characteristics of chemical and fluorescent parameters of water-extractable organic matter[J]. Chemosphere, 2016, 155: 358-366. DOI:10.1016/j.chemosphere.2016.04.051 |
| [58] |
ZHANG S, CHEN Z, WEN Q, et al. Effectiveness of bulking agents for co-composting penicillin mycelial dreg(PMD)and sewage sludge in pilot-scale system[J]. Environmental Science and Pollution Research, 2016, 23(2): 1362-1370. DOI:10.1007/s11356-015-5357-y |
| [59] |
CHEN Z, ZHANG S, WEN Q, et al. Effect of aeration rate on composting of penicillin mycelial dreg[J]. Journal of Environmental Sciences, 2015, 37: 172-178. DOI:10.1016/j.jes.2015.03.020 |
| [60] |
肖祖飞, 李刚, 陈欣瑶, 等. 不同类型外加碳源对制药污泥堆肥过程中青霉素降解的影响[J]. 环境化学, 2018, 37(8): 1728-1737. XIAO Z F, LI G, CHEN X Y, et al. Influence of carbon-rich amendments on penicillin degradation during pharmaceutical sewage sludge composting[J]. Environmental Chemistry, 2018, 37(8): 1728-1737. |
| [61] |
YANG L, ZHANG S, CHEN Z, et al. Maturity and security assessment of pilot-scale aerobic co-composting of penicillin fermentation dregs(PFDs)with sewage sludge[J]. Bioresource Technology, 2016, 204: 185-191. DOI:10.1016/j.biortech.2016.01.004 |
| [62] |
ZHAO J, SUN X, MUKESH K A, et al. Performance evaluation of gaseous emissions and Zn speciation during Zn-rich antibiotic manufacturing wastes and pig manure composting[J]. Bioresource Technology, 2018, 267: 688-695. DOI:10.1016/j.biortech.2018.07.088 |
| [63] |
DING N, LI W, LIU C, et al. Decline in extractable kitasamycin during the composting of kitasamycin manufacturing waste with dairy manure and sawdust[J]. Journal of Environmental Management, 2014, 134: 39-46. DOI:10.1016/j.jenvman.2013.12.030 |
| [64] |
LIAO H, ZHAO Q, CUI P, et al. Efficient reduction of antibiotic residues and associated resistance genes in tylosin antibiotic fermentation waste using hyperthermophilic composting[J]. Environment International, 2019, 133: 105203. DOI:10.1016/j.envint.2019.105203 |
| [65] |
ZHANG B, WANG M M, WANG B, et al. The effects of bio-available copper on macrolide antibiotic resistance genes and mobile elements during tylosin fermentation dregs co-composting[J]. Bioresource Technology, 2018, 251: 230-237. DOI:10.1016/j.biortech.2017.12.051 |
| [66] |
YANG M, MA X, XIE D, et al. A study towards minimising tylosin concentration and antibiotic resistance genes in tylosin fermentation dreg fertilizer[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104372. DOI:10.1016/j.jece.2020.104372 |
| [67] |
NAO K I, TAKEKI M, MISAKI S, et al. Tylosin degradation during manure composting and the effect of the degradation byproducts on the growth of green algae[J]. Science of the Total Environment, 2020, 718: 137295. DOI:10.1016/j.scitotenv.2020.137295 |
| [68] |
ŽIŽEK S, DOBEIC M, PINTARIČ Š, et al. Degradation and dissipation of the veterinary ionophore lasalocid in manure and soil[J]. Chemosphere, 2015, 138: 947-951. DOI:10.1016/j.chemosphere.2014.12.032 |
| [69] |
HAHN L, PADILHA M T S, PADILHA J C F, et al. Persistence of pathogens and the salinomycin antibiotic in composting piles of poultry litter[J]. Archivos de Zootecnia, 2012, 61(234): 279-285. DOI:10.4321/S0004-05922012000200012 |
| [70] |
RAMASWAMY J, PRASHER S O, PATEL R M, et al. The effect of composting on the degradation of a veterinary pharmaceutical[J]. Bioresource Technology, 2010, 101(7): 2294-2299. DOI:10.1016/j.biortech.2009.10.089 |
| [71] |
LIU Y, FENG Y, CHENG D, et al. Gentamicin degradation and changes in fungal diversity and physicochemical properties during composting of gentamicin production residue[J]. Bioresource Technology, 2017, 244: 905-912. DOI:10.1016/j.biortech.2017.08.057 |
| [72] |
ZHENG G, YU B, WANG Y, et al. Removal of triclosan during wastewater treatment process and sewage sludge composting: A case study in the middle reaches of the Yellow River[J]. Environment International, 2020, 134: 105300. DOI:10.1016/j.envint.2019.105300 |
| [73] |
WANG J, GU J, WANG X, et al. Enhanced removal of antibiotic resistance genes and mobile genetic elements during swine manure composting inoculated with mature compost[J]. Journal of Hazardous Materials, 2021, 411: 125135. DOI:10.1016/j.jhazmat.2021.125135 |
| [74] |
WEI H, MA J I, SU Y, et al. Effect of nutritional energy regulation on the fate of antibiotic resistance genes during composting of sewage sludge[J]. Bioresource Technology, 2020, 297: 122513. DOI:10.1016/j.biortech.2019.122513 |
| [75] |
GUO H, GU J, WANG X, et al. Responses of antibiotic and heavy metal resistance genes to bamboo charcoal and bamboo vinegar during aerobic composting[J]. Environmental Pollution, 2019, 252: 1097-1105. DOI:10.1016/j.envpol.2019.05.014 |
| [76] |
HUANG X, TIAN S, ZHENG J, et al. Fitness reduction of antibiotic resistome by an extra carbon source during swine manure composting[J]. Environmental Pollution, 2021, 277: 116819. DOI:10.1016/j.envpol.2021.116819 |
| [77] |
LIAO H, LU X, RENSING C, et al. Hyperthermophilic composting accelerates the removal of antibiotic resistance genes and mobile genetic elements in sewage sludge[J]. Environmental Science & Technology, 2018, 52: 266-276. |
| [78] |
LIU Y, FENG Y, CHENG D, et al. Dynamics of bacterial composition and the fate of antibiotic resistance genes and mobile genetic elements during the co-composting with gentamicin fermentation residue and lovastatin fermentation residue[J]. Bioresource Technology, 2018, 261: 249-256. DOI:10.1016/j.biortech.2018.04.008 |
| [79] |
CHANG J, JIANG T, ZHAO M, et al. Variation pattern of antibiotic resistance genes and microbial community succession during swine manure composting under different aeration strategies[J]. Journal of Chemical Technology & Biotechnology, 2020, 95(2): 466-473. |
| [80] |
武晋萍, 陈建文, 刘勇, 等. 鸡粪与中药渣共堆肥对抗生素抗性基因的影响[J]. 环境科学, 2019, 40(7): 3276-3284. WU J P, CHEN J W, LIU Y, et al. Effect of co- composting of chicken manure with Chinese medicinal herbal residues on antibiotic resistance genes[J]. Environmental Science, 2019, 40(7): 3276-3284. |
| [81] |
REN J, DENG L, LI C, et al. Effects of added thermally treated penicillin fermentation residues on the quality and safety of composts[J]. Journal of Environmental Management, 2021, 283: 111984. DOI:10.1016/j.jenvman.2021.111984 |
| [82] |
SUN X, ZHAO J, WANG Q, et al. Behaviors and related mechanisms of Zn resistance and antibiotic resistance genes during co-composting of erythromycin manufacturing wastes and pig manure[J]. Bioresource Technology, 2020, 318: 124048. DOI:10.1016/j.biortech.2020.124048 |
| [83] |
ZHANG B, YUAN Q, WANG M M, et al. Insights into the effects of Zn exposure on the fate of tylosin resistance genes and dynamics of microbial community during co-composting with tylosin fermentation dregs and swine manure[J]. Environmental Science and Pollution Research International, 2020, 28: 14423-14433. |
| [84] |
MA C, PO K L, XU J, et al. Molecular mechanisms underlying lignocellulose degradation and antibiotic resistance genes removal revealed via metagenomics analysis during different agricultural wastes composting[J]. Bioresource Technology, 2020, 314: 123731. DOI:10.1016/j.biortech.2020.123731 |
| [85] |
DUAN M, GU J, WANG X, et al. Effects of genetically modified cotton stalks on antibiotic resistance genes, intI1, and intI2 during pig manure composting[J]. Ecotoxicology and Environmental Safety, 2018, 147: 637-642. DOI:10.1016/j.ecoenv.2017.09.023 |
| [86] |
ZACHERY R S, AMY M S, BRYAN W, et al. Corn stalk residue may add antibiotic-resistant bacteria to manure composting piles[J]. Journal of Environmental Quality, 2020, 49(3): 20017. |
| [87] |
GOU C, WANG Y, ZHANG X, et al. Effects of chlorotetracycline on antibiotic resistance genes and the bacterial community during cattle manure composting[J]. Bioresource Technology, 2021, 323: 124517. DOI:10.1016/j.biortech.2020.124517 |
| [88] |
LI H, CHENG W, LI B, et al. The fate of antibiotic resistance genes during co-composting of swine manure with cauliflower and corn straw[J]. Bioresource Technology, 2020, 300: 122669. DOI:10.1016/j.biortech.2019.122669 |
| [89] |
DEVIN B H, HAO X, EDWARD T, et al. Effect of co-composting cattle manure with construction and demolition waste on the archaeal, bacterial, and fungal microbiota, and on antimicrobial resistance determinants[J]. PLoS One, 2017, 11(6): 0157539. |
| [90] |
GUO W, HUANG C, XI B, et al. The maturity period is the main stage of antibiotic resistance genes reduction in aerobic composting process of swine manure in sub-scale farms[J]. Bioresource Technology, 2021, 319: 124139. DOI:10.1016/j.biortech.2020.124139 |
| [91] |
LIU N, HAN H, YIN H, et al. Variations in the fate and risk analysis of amoxicillin and its degradation products during pig manure aerobic composting[J]. Journal of Hazardous Materials, 2018, 346: 234-241. DOI:10.1016/j.jhazmat.2017.11.050 |
| [92] |
CUI E, WU Y, ZUO Y, et al. Effect of different biochars on antibiotic resistance genes and bacterial community during chicken manure composting[J]. Bioresource Technology, 2016, 203: 11-17. DOI:10.1016/j.biortech.2015.12.030 |
| [93] |
PENG H, GU J, WANG X, et al. Insight into the fate of antibiotic resistance genes and bacterial community in co-composting green tea residues with swine manure[J]. Journal of Environmental Management, 2020, 266: 110581. DOI:10.1016/j.jenvman.2020.110581 |
| [94] |
LI Y, LIU B, ZHANG X, et al. Effects of Cu exposure on enzyme activities and selection for microbial tolerances during swine-manure composting[J]. Journal of Hazardous Materials, 2015, 283: 512-518. DOI:10.1016/j.jhazmat.2014.09.061 |
| [95] |
QIU X, ZHOU G, WANG H, et al. The behavior of antibiotic-resistance genes and their relationships with the bacterial community and heavy metals during sewage sludge composting[J]. Ecotoxicology and Environmental Safety, 2021, 216: 112190. DOI:10.1016/j.ecoenv.2021.112190 |
| [96] |
CUI P, BAI Y, LI X, et al. Enhanced removal of antibiotic resistance genes and mobile genetic elements during swine manure composting covered with a semi-permeable membrane[J]. Journal of Hazardous Materials, 2020, 396: 122738. DOI:10.1016/j.jhazmat.2020.122738 |
| [97] |
CAO R, BEN W, QIANG Z, et al. Removal of antibiotic resistance genes in pig manure composting influenced by inoculation of compound microbial agents[J]. Bioresource Technology, 2020, 317: 123966. DOI:10.1016/j.biortech.2020.123966 |
| [98] |
DUAN M, ZHANG Y, ZHOU B, et al. Changes in antibiotic resistance genes and mobile genetic elements during cattle manure composting after inoculation with Bacillus subtilis[J]. Bioresource Technology, 2019, 292: 122011. DOI:10.1016/j.biortech.2019.122011 |
| [99] |
HU T, WANG X, ZHENG L, et al. Effects of inoculation with lignocellulose-degrading microorganisms on antibiotic resistance genes and the bacterial community during co-composting of swine manure with spent mushroom substrate[J]. Environmental Pollution, 2019, 25: 110-118. |
| [100] |
GUO H, GU J, WANG X, et al. Elucidating the effect of microbial inoculum and ferric chloride as additives on the removal of antibiotic resistance genes from chicken manure during aerobic composting[J]. Bioresource Technology, 2020, 309: 122802. DOI:10.1016/j.biortech.2020.122802 |
| [101] |
QIAN X, GU J, SUN W, et al. Effects of passivators on antibiotic resistance genes and related mechanisms during composting of copper-enriched pig manure[J]. Science of the Total Environment, 2019, 674: 383-391. DOI:10.1016/j.scitotenv.2019.04.197 |
| [102] |
CUI E, WU Y, JIAO Y, et al. The behavior of antibiotic resistance genes and arsenic influenced by biochar during different manure composting[J]. Environmental Science and Pollution Research International, 2017, 24(16): 14484-14490. DOI:10.1007/s11356-017-9028-z |
| [103] |
WANG J, SUI B, SHEN Y, et al. Effect of different biochars on antibiotic resistance genes during swine manure thermophilic composting[J]. International Journal of Agricultural and Biological Engineering. |
| [104] |
LI H, DUAN M, GU J, et al. Effects of bamboo charcoal on antibiotic resistance genes during chicken manure composting[J]. Ecotoxicology and Environmental Safety, 2017, 140: 1-6. DOI:10.1016/j.ecoenv.2017.01.007 |
| [105] |
PENG S, LI H, SONG D, et al. Influence of zeolite and superphosphate as additives on antibiotic resistance genes and bacterial communities during factory-scale chicken manure composting[J]. Bioresource Technology, 2018, 263: 393-401. DOI:10.1016/j.biortech.2018.04.107 |
| [106] |
ZHANG J, CHEN M, SUI Q, et al. Impacts of addition of natural zeolite or a nitrification inhibitor on antibiotic resistance genes during sludge composting[J]. Water Research, 2016, 91: 339-349. DOI:10.1016/j.watres.2016.01.010 |
| [107] |
CAO Y, HU H W, GUO H G, et al. Lignite as additives accelerates the removal of antibiotic resistance genes during poultry litter composting[J]. Bioresource Technology, 2020, 315: 123841. DOI:10.1016/j.biortech.2020.123841 |
| [108] |
WANG R, ZHANG J, SUI Q, et al. Effect of red mud addition on tetracycline and copper resistance genes and microbial community during the full scale swine manure composting[J]. Bioresource Technology, 2016, 216: 1049-1057. DOI:10.1016/j.biortech.2016.06.012 |
| [109] |
ZHAO W, GU J, WANG X, et al. Effects of shrimp shell powder on antibiotic resistance genes and the bacterial community during swine manure composting[J]. Science of the Total Environment, 2021, 752: 142162. DOI:10.1016/j.scitotenv.2020.142162 |
| [110] |
SONG T, ZHU C, XUE S, et al. Comparative effects of different antibiotics on antibiotic resistance during swine manure composting[J]. Bioresource Technology, 2020, 315: 123820. DOI:10.1016/j.biortech.2020.123820 |
| [111] |
BAO J, WANG X, GU J, et al. Effects of macroporous adsorption resin on antibiotic resistance genes and the bacterial community during composting[J]. Bioresource Technology, 2020, 295: 121997. DOI:10.1016/j.biortech.2019.121997 |
| [112] |
GUO A, GU J, WANG X, et al. Effects of superabsorbent polymers on the abundances of antibiotic resistance genes, mobile genetic elements, and the bacterial community during swine manure composting[J]. Bioresource Technology, 2017, 244: 658-663. DOI:10.1016/j.biortech.2017.08.016 |
| [113] |
ZHANG Y, LI H, GU J, et al. Effects of adding different surfactants on antibiotic resistance genes and intI1 during chicken manure composting[J]. Bioresource Technology, 2016, 219: 545-551. DOI:10.1016/j.biortech.2016.06.117 |
| [114] |
WANG Q, GU J, WANG X, et al. Effects of nano-zerovalent iron on antibiotic resistance genes and mobile genetic elements during swine manure composting[J]. Environmental Pollution, 2020, 258: 113654. DOI:10.1016/j.envpol.2019.113654 |
| [115] |
LIU Z, WANG X, WANG F, et al. The progress of composting technologies from static heap to intelligent reactor: Benefits and limitations[J]. Journal of Cleaner Production, 2020, 270: 122328. DOI:10.1016/j.jclepro.2020.122328 |
| [116] |
GONG P, LIU H, XIN Y, et al. Composting of oxytetracycline fermentation residue in combination with hydrothermal pretreatment for reducing antibiotic resistance genes enrichment[J]. Bioresource Technology, 2020, 318: 124271. DOI:10.1016/j.biortech.2020.124271 |
| [117] |
FAN H, WU S, JOHN W, et al. Effective removal of antibiotic resistance genes andpotential links with archaeal communities during vacuum-type composting and positive-pressure composting[J]. Journal of Environmental Sciences, 2020, 89: 277-286. DOI:10.1016/j.jes.2019.09.006 |
| [118] |
CAO R, WANG J, BEN W, et al. The profile of antibiotic resistance genes in pig manure composting shaped by composting stage: Mesophilic-thermophilic and cooling-maturation stages[J]. Chemosphere, 2020, 250: 126181. DOI:10.1016/j.chemosphere.2020.126181 |
 2021, Vol. 40
2021, Vol. 40