文章信息
- 袁哲军, 张洪昌, 胡双庆, 沈根祥, 钱晓雍, 王振旗, 朱英, 张玉
- YUAN Zhe-jun, ZHANG Hong-chang, HU Shuang-qing, SHEN Gen-xiang, QIAN Xiao-yong, WANG Zhen-qi, ZHU Ying, ZHANG Yu
- 上海典型畜禽场周边河流雌激素污染特征研究
- Study on estrogen pollution characteristics of rivers around typical livestock and poultry farms in Shanghai
- 农业环境科学学报, 2017, 36(8): 1583-1589
- Journal of Agro-Environment Science, 2017, 36(8): 1583-1589
- http://dx.doi.org/10.11654/jaes.2017-0325
文章历史
- 收稿日期: 2017-03-10
2. 上海市环境科学研究院, 上海 200233;
3. 华东理工大学资源与环境工程学院, 上海 200237
2. Shanghai Academy of Environmental Sciences, Shanghai 200233, China;
3. College of Resources and Environmental Engineering, East China University of Science and Technology, Shanghai 200237, China
人们生活水平的提高带动了畜禽养殖业向规模化和集约化方向快速发展,与此同时也产生了大量畜禽粪便。研究表明,目前我国的畜禽养殖污染防治措施仍不完善[1],环境中90%的天然雌激素均来自畜禽养殖业排放的畜禽粪便[2],包括雌酮(Estrone,E1)、雌二醇(17β-estradiol,E2)和雌三醇(Estriol,E3)。而人工合成雌激素,如乙炔基雌二醇(17β-ethinylestradiol,EE2)和己烯雌酚(Diethylstilbestrol,DES),因其具有促进动物生长的作用,被用作激素类药物或动物饲料添加剂应用到畜禽养殖业。这些天然和人工合成雌激素具有很强的内分泌干扰活性,应该被优先关注[3]。它们能够造成河流中鱼类胚胎发育迟滞和畸形[4],能够在ng·L-1痕量水平诱导雄鱼雌性化[5-8]。由此可见,畜禽养殖排放的雌激素对水生环境的污染风险不容忽视。
上海市规模化畜禽养殖场产生的畜禽粪污主要采用资源化还田模式进行处理[9],然而研究表明,畜禽粪污通过堆肥处理和污水处理设施处理后其自由态雌激素并不能被完全去除[10-13],粪污中的天然雌激素会通过农田施肥和地表径流等作用进入周边水体[14-16]。由于上海市河流体系复杂,支流纵横交错,尤其小河、沟渠数量众多,也加剧了水环境中雌激素污染防治的难度,因此更应当引起足够重视。
本研究利用固相萃取-高效液相色谱-串联质谱(SPE-HPLC-MS/MS)方法对典型畜禽养殖场周边河流地表水中5种天然和人工合成雌激素E1、E2、E3、EE2和DES进行定量检测,同时分析其污染来源和空间分布特征,并利用雌二醇当量(EEQ)评价雌激素活性,旨在为畜禽养殖雌激素污染危害评价和风险管控提供科学依据。
1 材料与方法 1.1 试剂与仪器仪器:AB5500Q-trap高效液相色谱串联质谱仪(美国AB公司),配岛津30A液相色谱。12通道固相萃取装置(美国Waters公司),Oasis HLB固相萃取柱(200 mg,6 mL,Waters公司),Milli-Q超纯水器(美国Millipore公司),氮吹仪(Anpel 12通道,安谱实验科技股份有限公司),超声波清洗器(KQ3200E,昆山市超声仪器有限公司),pH计(S220,梅特勒-托多仪器有限公司),溶解氧测定仪(YSI-58,YSI Instruments),便携式悬浮物测定仪(PSS-200型,深圳市昌鸿科技有限公司)。
试剂:甲醇、乙腈均为HPLC级,购自美国Tedia公司。E1(99.3%)、E2(97.5%)、E3(99.5%)、EE2(99.0%)、DES(99.5%)均购自德国Dr. Ehrenstorfer公司。上述标准品均用甲醇配成标准储备液,并配制浓度为100 mg·L-1的5种雌激素的混合标准溶液。根据需要用甲醇配制成系列浓度的标准工作溶液,于4 ℃冰箱中保存。
1.2 样品的采集与前处理采集上海市三个规模化畜禽养殖场周边河流上下游各500 m处的断面地表水样,共6个断面,每个断面沿水体截面设置3个采样点,每个采样点取4 L水样,保存于4 L棕色瓶内,并立即加入4 mol·L-1的盐酸防止微生物对目标物质的降解。水样快速运回实验室放于4 ℃冰库避光保存,48 h内处理水样。本次采样时间为冬季,各采样点的水温范围为11.0~12.5 ℃,pH 7.50~7.70,水样均呈弱碱性。溶解氧(DO)为4.5~7.0 mg·L-1,悬浮物(SS)浓度为10.2~23.3 mg·L-1。
每个采样点设置3个平行样,每份水样取1 L,经玻璃纤维滤纸(孔径0.7 μm)过滤后,加入50 ng内标物质E2-d5,调pH值为3。固相萃取方法在谭丽超等[17]方法的基础上有所改进,依次用6 mL甲醇、6 mL水活化HLB固相萃取小柱。上样速率为2~3 mL·min-1,上样结束后,依次用5 mL超纯水、5 mL含5%甲醇水溶液淋洗小柱,减压抽干10 min,最后用10 mL甲醇分两次洗脱。洗脱液在40 ℃条件下氮吹至近干,用1 mL甲醇/水(体积比为7/3)重新溶解,过0.22 μm微孔滤膜到进样瓶中,进行HPLC-MS/MS测定。
1.3 HPLC-MS/MS分析液相色谱条件:Shim-Pack XR-ODSII C18色谱柱(100 mm ×2 mm,1.0 μm);柱温40 ℃;进样量5 μL;进样室温度10 ℃;流动相流速0.3 mL·min-1。流动相A为Milli-Q超纯水,B为乙腈。梯度洗脱程序:0~3.0 min,30%B;3.0~4.0 min,30%B~90%B;4.0~7.0 min,90%B;7.0~8.0 min,90%B~30%B;8.0~10.0 min,30%B。
质谱条件:采用电喷雾离子源负离子(ESI-)模式,离子源温度为550 ℃,离子化电压为-4500 V,气帘气(CUR)为35 psi(1 psi=6.895 kPa),喷雾气(GS1)为50 psi,辅助加热气(GS2)为50 psi,碰撞气(CAD)为Medium,(碰撞气为高纯氮气)。采用多反应监测模式(MRM)进行检测。
1.4 质量保证和质量控制配制5种雌激素的系列混合标准溶液,浓度范围为1.0~1000 μg·L-1,采用内标法定量,在选定的色谱和质谱条件下进行测定,以目标物和内标物浓度的比值为横坐标,以其峰面积的比值为纵坐标,得到5种雌激素的决定系数(r2)均大于0.996(表 1),表明所建立的标准曲线线性关系良好。在该方法条件下,以3倍和10倍信噪比(S/N)对应的样品中雌激素浓度为方法检测限(LOD)和定量限(LOQ),获得各目标物的LOD范围为0.20~1.71 ng·L-1,LOQ范围为0.62~5.25 ng·L-1。

|
选择两种不同水样(Milli-Q超纯水和养殖场周边河流地表水样),准确添加5种雌激素的混合标准溶液,配制成100 ng·L-1的加标水样,进行加标回收率试验。按上述前处理方法进行萃取、净化和浓缩,用HPLC-MS/MS测定各目标物的浓度,并计算回收率与相对标准偏差,得到Milli-Q超纯水样中目标物回收率为76.5%~92.1%,相对标准偏差(RSD)小于6.0%;养殖场周边河流地表水样中目标物回收率为67.8%~98.4%(表 1),相对标准偏差(RSD)小于10.0%,表明所建立的方法具有良好的准确度和精密度。
1.5 数据处理采用EEQ表征雌激素活性,其计算公式[18]如下,其中雌二醇当量因子(EEF)参考隋倩等[19]的方法,即采用体外法测试所得EEF的最大值进行计算,雌激素E1、E2、E3、EE2和DES对应的EEF分别为0.59、1.0、0.26、8.71、8.0。
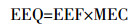
式中:MEC为各雌激素实测浓度,ng·L-1。
取三次平行试验的算术平均值,利用Excel 2010进行数据计算及处理。同时利用t检验对各样品数据进行显著性分析。
2 结果与讨论 2.1 畜禽场周边河流地表水中雌激素的分布特征对水样中5种天然和人工合成雌激素进行检测,结果如表 2所示。E1、E2和E3的检出率均为100%,而EE2和DES检出率分别为94.44%和27.78%。雌激素E1、E2、E3、EE2和DES的浓度范围分别为21.66~73.40、8.75~55.96、4.56~23.90、ND~19.42、ND~3.23 ng·L-1。在已有研究中,Chen等[20]对集中式畜禽养殖场附近受纳水体中的雌激素进行调查发现,E1、E2、E3和DES的平均浓度分别为7.4~1267、ND~313.6、ND~210、ND~3.3 ng·L-1,而EE2低于检出限。Lei等[21]对天津地区某三条河流中雌激素浓度进行了测定,结果显示E1、E2、E3、EE2和DES的检出浓度分别为0.64~55.33、ND~32.4、ND~46.4、ND~35.6、ND~8.51 ng·L-1。综合以上数据可以看出,本研究中河流地表水中雌激素浓度与上述其他水环境处于同一数量级。相关报道显示,天然雌激素主要通过污水处理厂和畜禽养殖厂粪便处理单元进入环境当中,并且畜禽养殖排放的雌激素量约为污水处理厂排放量的10倍,是最重要的雌激素排放源[13, 22]。可见,调查水体中高检出率的雌激素与周边的畜禽养殖场可能存在密切关系。

|
图 1显示三个畜禽场上下游地表水中5种雌激素的组成和分布情况。与其他4种雌激素相比,E1在猪场上游、猪场下游和鸡场上游、鸡场下游水体中所占比例(50.48%、55.93%和40.21%、56.67%)均最高。有研究表明,水中的E1浓度之所以较高,可能因为其自身的稳定性很高,或是E1的葡萄糖醛酸结合态水解产生自由态的E1,或是E2氧化降解产生了E1[23]。目前很多文献也报道了地表水体中雌激素E1的浓度高于E2和E3[20-21, 24]。而在奶牛场上下游水体中,雌激素E2所占比例最高,与黄浦江上游养殖区周边水体中雌激素的检出情况一致[25]。

|
| 图 1 畜禽场上下游河道中雌激素的分布特征 Figure 1 Estrogens distribution pattern in the upstream and downstream of livestock and poultry farms |
比较各养殖场周边河流上下游水体中雌激素总量可以看出,上游河段雌激素总量表现为奶牛场(75.43 ng·L-1)>猪场(66.72 ng·L-1)>鸡场(61.72 ng·L-1),而下游河段雌激素总量表现为猪场(118.39 ng·L-1)>奶牛场(96.43 ng·L-1)>鸡场(58.47 ng·L-1)。鸡场上游和下游水体中雌激素总量均最低,表明猪场和奶牛场周边河流受雌激素污染程度高于鸡场周边河流。与上游相比,猪场和奶牛场下游的雌激素总量分别增加了43.6%和21.8%,而鸡场下游的雌激素总量下降了5.3%,表明猪场和奶牛场排污对周边水体雌激素污染的贡献大。这与Zhang等[26]研究中猪和牛的雌激素排放量显著高于鸡的排放量结果一致。以上数据显示,各畜禽场周边河流上游水体中雌激素含量很高,表明该地区几条河流中的雌激素并非完全由这三个畜禽场排污产生,可能存在其他排放源尚需进一步追溯。
2.2 猪场周边河流地表水中雌激素的污染状况如图 2所示,猪场上下游水体中均检出了E1、E2、E3和EE2,其中E1平均浓度(49.95 ng·L-1)是E2平均浓度(14.30 ng·L-1)的3倍左右,而DES未检出。与上游相比,猪场下游E1含量增加96.6%,达到极显著水平(P<0.01),E2含量增加42.6%,达到显著水平(P<0.05),E3和EE2含量虽有所增加,但含量间无显著差异(P>0.05)。这可能是因为猪主要通过尿液排放雌激素(98%~99%)[26-27],而E1是尿液中主要的自由态雌激素[28]。数据分析表明,该养猪场对周边河流水体造成了雌激素污染,成为了天然雌激素E1和E2的主要排放源。EE2含量的增加表明该养猪场可能使用了含EE2的饲料添加剂。虽然EE2作为动物饲料添加剂已被欧盟和我国禁止使用[29],但在国内一些畜禽养殖场排放的畜禽粪污中仍能检出EE2,如Liu等[30]在猪场冲洗水中检测出高浓度的EE2,Zhang等[26]在猪场的氧化塘中也发现了高含量的EE2。

|
| *,**表示各雌激素下游浓度和上游浓度相比的显著性概率:*表示差异显著(P<0.05),**表示差异极显著(P<0.01)。下同 *, ** indicate significant probability of concentration of each estrogen between the downstream and the upstream:* indicate significant difference(P < 0.05), ** indicate extremely significant difference(P < 0.01). The same below 图 2 猪场上下游水体中5种雌激素的检出浓度 Figure 2 Concentration of 5 estrogens in the upstream and downstream of swine farm |
鸡场上下游水体中雌激素的检出情况同猪场相似,E1、E2、E3和EE2均被检出,而未检出DES。鸡场上下游水体中雌激素的组成和浓度如图 3所示。上游水体中雌激素浓度表现为E1>E2>E3>EE2,浓度范围为2.14~27.50 ng·L-1;下游水体呈现为E1>E3>E2>EE2,浓度范围为1.98~38.45 ng·L-1。与上游相比,下游E2的浓度降低40%,表现出极显著差异(P<0.01),E3的浓度降低26%,表现出显著性差异(P<0.05),E1的含量虽有所增加,但差异不显著。相关研究表明,家禽相较于猪和牛所产生的雌激素量较少[26],并且鸡主要排放粪便,现场调研时发现,该养鸡场的粪便处理方式主要是高温堆肥,堆肥产品主要用于商业出售,故对周边河流产生的雌激素污染较小。相关研究发现,随着水流的稀释作用和雌激素自身的降解作用,下游雌激素浓度较上游会出现明显降低现象[20]。另外,雌激素是中等极性类物质(lg Kow 2.6~4.0),吸附能力较强,水体中的雌激素易吸附到悬浮颗粒物上或迁移到沉积物中[2, 31]。这也可能是该河流地表水中雌激素浓度降低的一个重要原因。而E1含量的增加很可能与结合态雌激素的水解以及E2的降解有关[23]。

|
| 图 3 鸡场上下游水体中5种雌激素的检出浓度 Figure 3 Concentration of 5 estrogens in the upstream and downstream of chicken farm |
奶牛场周边河流雌激素污染特征与猪场和鸡场有所差异。如图 4所示,上下游水体中5种雌激素均被检出,且雌激素E1和E2检出浓度相对较高。与上游相比,下游天然雌激素E2含量增加45.1%,表现出极显著差异(P<0.01),E1和E3含量略有增加(9.0%和6.5%),但差异不显著(P>0.05);人工合成雌激素EE2含量增加33.5%,DES含量下降17.7%,但均无显著性差异(P>0.05)。相关研究发现,在上海地区多个奶牛场新鲜粪便、尿液、氧化塘污水和厌氧污水池中均检出一定浓度的天然雌激素,且厌氧污水池对雌激素的去除效率低于50%[26]。相关研究表明,在某奶牛养殖场周边河道地表水中检测到显著的雌激素活性,其结果表明该养殖场对其周边水体造成了雌激素污染[32]。本研究通过现场调研发现,奶牛场污水经厌氧池处理后通过污水管道就近还田,若厌氧池未能将粪污中的雌激素完全去除,则很可能污染还田土壤和周边水体。综合分析表明,该奶牛场对周边水体造成了雌激素污染,且主要雌激素污染种类为E2,而这与Hanselman等[31]和Combalbert等[33]所述奶牛排泄物中最主要的雌激素是E1和E2的结论并不完全相符。这可能与养殖场污水处理设施、粪便处理方式、雌激素去除效率以及其他影响因素有关。

|
| 图 4 奶牛场上下游水体中5种雌激素的检出浓度 Figure 4 Concentration of 5 estrogens in the upstream and downstream of dairy farm |
采用EEQ表征各雌激素污染物活性,其计算结果见表 3。畜禽场周边河流中EEQ浓度范围为54.15~194.61 ng·L-1。而相关研究报道,台湾某受畜禽场污染地表水体中3种雌激素总EEQ浓度范围为17.3~137.9 ng·L-1[20],日本某受养殖场污水影响的河流中两种雌激素总EEQ浓度范围为1.50~24.60 ng·L-1[34],黄浦江上游水源地干支流区水体中6种雌激素总EEQ浓度范围为32~1 007.3 ng·L-1[25]。通过对比可以看出,被调查河流水体中雌激素活性处于中等水平。三个畜禽场周边河流中EEQ总体表现为猪场(149.42 ng·L-1)>奶牛场(133.59 ng·L-1)>鸡场(55.77 ng·L-1),表明猪场周边河流地表水中的雌激素活性最大,奶牛场次之,鸡场最小。这与上述分析结果一致,均表明猪场和奶牛场周边河流地表水受雌激素污染程度较高。由表 3数据可以看出,EE2雌激素活性高于其他雌激素,其在所有采样断面中对总EEQ的贡献均最大,故调查河流水体存在的EE2污染风险不容忽视。
研究表明,为了评估E2的存在对水体鱼类繁殖能力所产生的影响,建议E2的最低观察效应浓度(LOEC)是10 ng·L-1[35]。根据欧盟关于污染物生态风险的安全系数设定[36],将引起雌激素内分泌干扰效应的标准定为1 ng·L-1,即凡雌激素污染物的EEQ大于1 ng·L-1的物质即被认为会对受纳水体水生生物产生内分泌干扰作用。结合表 3可知,三个典型养殖场周边河流上下游地表水中EEQ均显著高于10 ng·L-1,表明雌激素对水生生物均存在不同程度的威胁,雌激素污染风险应引起足够重视。
3 结论(1)天然雌激素E1、E2和E3在各个采样点均被检出,EE2只有一个采样点未被检出,而DES只在奶牛场上下游采样点被检出。三个典型养殖场周边河流地表水体均受到不同程度的雌激素污染,且猪场和奶牛场周边河流受污染程度高于鸡场周边河流。
(2)典型猪场和典型奶牛场是E2的主要排放源,典型猪场是E1的主要排放源,而典型鸡场对其周边河流产生的雌激素污染较小。
(3)典型畜禽养殖场周边河流中雌激素活性表现为猪场>奶牛场>鸡场,三条河流均存在一定程度的雌激素污染风险。
| [1] |
吴根义, 廖新俤, 贺德春, 等. 我国畜禽养殖污染防治现状及对策[J]. 农业环境科学学报, 2014, 33(7): 1261-1264. WU Gen-yi, LIAO Xin-di, HE De-chun, et al. Current situation and countermeasures of livestock industry pollution control in China[J]. Journal of Agro-Environment Science, 2014, 33(7): 1261-1264. DOI:10.11654/jaes.2014.07.001 |
| [2] |
Khanal S K, Xie B, Thompson M L, et al. Fate, transport, and biodegradation of natural estrogens in the environment and engineered systems[J]. Environmental Science & Technology, 2006, 40(21): 6537-6546. |
| [3] |
Ying G G, Kookana R S, Ru Y J. Occurrence and fate of hormone steroids in the environment[J]. Environment International, 2002, 28(6): 545-551. DOI:10.1016/S0160-4120(02)00075-2 |
| [4] |
Hu S Q, Zhang H C, Shen G X, et al. Effects of 17β-estradiol and 17α-ethinylestradiol on the embryonic development of the clearhead icefish(Protosalanx hyalocranius)[J]. Chemosphere, 2017, 176: 18-24. DOI:10.1016/j.chemosphere.2017.02.094 |
| [5] |
Lorenzen A, Hendel J G, Conn K L, et al. Survey of hormone activities in municipal biosolids and animal manures[J]. Environmental Toxicology, 2004, 19(3): 216-225. DOI:10.1002/tox.v19:3 |
| [6] |
Ternes T A, Stumpf M, Mueller J, et al. Behavior and occurrence of estrogens in municipal sewage treatment plants:Ⅰ. Investigations in Germany, Canada and Brazil[J]. Science of the Total Environment, 1999, 225(1/2): 81-90. |
| [7] |
Andersen L, Holbech H, Gessbo A, et al. Effects of exposure to 17α-ethinylestradiol during early development on sexual differentiation and induction of vitellogenin in zebrafish(Danio rerio)[J]. Comp Biochem Physiol C Toxicol Pharmacol, 2003, 134(3): 365-374. DOI:10.1016/S1532-0456(03)00006-1 |
| [8] |
Jobling S, Casey D, Rogersgray T, et al. Comparative responses of molluscs and fish to environmental estrogens and an estrogenic effluent[J]. Aquatic Toxicology, 2003, 65(2): 205-220. DOI:10.1016/S0166-445X(03)00134-6 |
| [9] |
王振旗, 钱晓雍, 沈根祥. 上海市规模化畜禽场污染减排模式分析与应用[J]. 农业环境科学学报, 2014, 33(10): 2030-2035. WANG Zhen-qi, QIAN Xiao-yong, SHEN Gen-xiang. Analysis and application of pollution reduction techniques for scale livestock and poultry farms in Shanghai[J]. Journal of Agro-Environment Science, 2014, 33(10): 2030-2035. DOI:10.11654/jaes.2014.10.021 |
| [10] |
Combalbert S, Bellet V, Dabert P, et al. Fate of steroid hormones and endocrine activities in swine manure disposal and treatment facilities[J]. Water Research, 2011, 46(3): 895-906. |
| [11] |
Gadd J B, Tremblay L A, Northcott G L. Steroid estrogens, conjugated estrogens and estrogenic activity in farm dairy shed effluents[J]. Environmental Pollution, 2010, 158(3): 730-736. DOI:10.1016/j.envpol.2009.10.015 |
| [12] |
Hutchins S R, White M V, Hudson F M, et al. Analysis of lagoon samples from different concentrated animal feeding operations for estrogens and estrogen conjugates[J]. Environmental Science & Technology, 2007, 41(3): 738-744. |
| [13] |
Raman D R, Williams E L, Layton A C, et al. Estrogen content of dairy and swine wastes[J]. Environmental Science & Technology, 2004, 38(13): 3567-3573. |
| [14] |
Bartelthunt S, Snow D D, Damonpowell T, et al. Occurrence of steroid hormones and antibiotics in shallow groundwater impacted by livestock waste control facilities[J]. Journal of Contaminant Hydrology, 2011, 123(3): 94-103. |
| [15] |
Belfroid A C, Van der Horst A, Vethaak A D, et al. Analysis and occurrence of estrogenic hormones and their glucuronides in surface water and waste water in the Netherlands[J]. Science of the Total Environment, 1999, 225(1/2): 101-108. |
| [16] |
Dutta S, Inamdar S, Tso J, et al. Dissolved organic carbon and estrogen transport in surface runoff from agricultural land receiving poultry litter[J]. Jawra Journal of the American Water Resources Association, 2012, 48(3): 558-569. DOI:10.1111/j.1752-1688.2011.00634.x |
| [17] |
谭丽超, 葛峰, 单正军, 等. 超高效液相色谱-串联质谱法测定污水处理厂水样中的雌激素[J]. 生态与农村环境学报, 2011, 27(5): 86-92. TAN Li-chao, GE Feng, SHAN Zheng-jun, et al. Determination of estrogens in water samples from wastewater treatment plant using ultra-performance liquid chromatography-electrospray tandem mass spectrometry method[J]. Journal of Ecology and Rural Environment, 2011, 27(5): 86-92. |
| [18] |
Korner W, Spengler P, Bolz U, et al. Substances with estrogenic activity in effluents of sewage treatment plants in southwestern Germany:2. Biological analysis[J]. Environmental Toxicology & Chemistry, 2001, 20(10): 2142-2151. |
| [19] |
隋倩, 黄俊, 余刚. 中国城市污水处理厂内分泌干扰物控制优先性分析[J]. 环境科学, 2009, 30(2): 384-390. SUI Qian, HUANG Jun, YU Gang. Priority analysis for controlling endocrine disrupting chemicals in municipal wastewater treatment plants of China[J]. Environmental Science, 2009, 30(2): 384-390. |
| [20] |
Chen T S, Chen T C, Yeh K J C, et al. High estrogen concentrations in receiving river discharge from a concentrated livestock feedlot[J]. Science of the Total Environment, 2010, 408(16): 3223-3230. DOI:10.1016/j.scitotenv.2010.03.054 |
| [21] |
Lei B L, Huang S B, Zhou Y Q, et al. Levels of six estrogens in water and sediment from three rivers in Tianjin area, China[J]. Chemosphere, 2009, 76: 36-42. DOI:10.1016/j.chemosphere.2009.02.035 |
| [22] |
李艳霞, 韩伟, 林春野, 等. 畜禽养殖过程中雌激素的排放及其环境行为[J]. 生态学报, 2010, 30(4): 1058-1065. LI Yan-xia, HAN Wei, LIN Chun-ye, et al. Excretion of estrogens in the livestock and poultry production and their environmental behaviors[J]. Acta Ecologica Sinica, 2010, 30(4): 1058-1065. |
| [23] |
Ternes T A, Kreckel P, Mueller J. Behaviour and occurrence of estrogens in municipal sewage treatment plants? Ⅱ. Aerobic batch experiments with activated sludge[J]. Science of the Total Environment, 1999, 225(1/2): 91-99. |
| [24] |
Furuichi T, Kannan K, Giesy J P, et al. Contribution of known endocrine disrupting substances to the estrogenic activity in Tama River water samples from Japan using instrumental analysis and in vitro reporter gene assay[J]. Water Research, 2004, 38(20): 4491-4501. DOI:10.1016/j.watres.2004.08.007 |
| [25] |
聂明华. 黄浦江上游水源地水体中雌激素的分布特征和分配机制[D]. 上海: 华东师范大学, 2012. NIE Ming-hua. Occurrence and phase distribution of selected estrogens in the water of the water source protection area of upper reach of Huangpu River[D]. Shanghai:East China Normal University, 2012. http://cdmd.cnki.com.cn/Article/CDMD-10269-1012434173.htm |
| [26] |
Zhang H, Shi J H, Liu X W, et al. Occurrence and removal of free estrogens, conjugated estrogens, and bisphenol A in manure treatment facilities in East China[J]. Water Research, 2014, 58(7): 248-257. |
| [27] |
Zhang H, Shi J H, Liu X W, et al. Occurrence of free estrogens, conjugated estrogens, and bisphenol A in fresh livestock excreta and their removal by composting in North China[J]. Environmental Science & Pollution Research, 2014, 21(16): 9939-9947. |
| [28] |
Johnson A C, Williams R J, Matthiessen P. The potential steroid hormone contribution of farm animals to freshwaters, the United Kingdom as a case study[J]. Science of the Total Environment, 2006, 362(1/2/3): 166-178. |
| [29] |
Durant A A, Fente C A, Franco C M, et al. Gas chromatography-tandem mass spectrometry determination of 17α-ethinylestradiol residue in the hair of cattle:Application to treated animals[J]. Journal of Agricultural & Food Chemistry, 2002, 50(3): 436-440. |
| [30] |
Liu S, Ying G G, Zhang R Q, et al. Fate and occurrence of steroids in swine and dairy cattle farms with different farming scales and wastes disposal systems[J]. Environmental Pollution, 2012, 170(8): 190-201. |
| [31] |
Hanselman T A, Graetz D A, Wilkie A C. Manure-borne estrogens as potential environmental contaminants:A review[J]. Environmental Science and Technology, 2003, 37(24): 5471-5478. DOI:10.1021/es034410+ |
| [32] |
Soto A M, Calabro J M, Prechtl N V, et al. Androgenic and estrogenic activity in water bodies receiving cattle feedlot effluent in eastern Nebraska, USA[J]. Environmental Health Perspectives, 2004, 112(3): 346-352. |
| [33] |
Combalbert S, Hernandezraquet G. Occurrence, fate, and biodegradation of estrogens in sewage and manure[J]. Applied Microbiology and Biotechnology, 2010, 86(6): 1671-1692. DOI:10.1007/s00253-010-2547-x |
| [34] |
Tashiro Y, Takemura A, Fujii H, et al. Livestock wastes as a source of estrogens and their effects on wildlife of Manko tidal flat, Okinawa[J]. Marine Pollution Bulletin, 2003, 47(1-6): 143-147. DOI:10.1016/S0025-326X(03)00053-5 |
| [35] |
Young W F, Whitehouse P, Johnson I, et al. Proposed predicted no effect concentrations(PNECs) for natural and synthetic steroid oestrogens in surface waters[R]. Environment Agency R&D Technical Report P2-T04/1. Bristol:England and Wales Environment Agency, 2004.
|
| [36] |
European Commission. Technical guidance document in support of commission directive 93/67/EEC on risk assessment for new notified substances and commission regulation(EC) No. 1488/94 on risk assessment for existing substances[R]. Luxembourg:Office for Official Publications of the European Communities, 1996:328-334.
|
 2017, Vol. 36
2017, Vol. 36





