2. 农业部江苏耕地保育科学观测实验站, 南京 210014
2. Scientific Observing and Experimental Station for Farmland Conservation(Jiangsu), Ministry of Agriculture, Nanjing 210014, China
秸秆还田是可持续农业生产中资源循环利用的重要措施之一,不仅能够改善土壤结构、增加土壤养分和提高作物产量[1-5],而且对土壤微生物也有着重要的影响[6-7]。古菌是土壤微生物的重要组成部分,直接影响土壤物质循环和养分转化等[8-10]。然而由于土壤中大部分古菌很难分离培养,采用传统的土壤微生物研究方法如微生物平板培养法、变性梯度凝胶电泳(DGGE)等往往无法详细描述出土壤微生物的群落多样性和组成方面的信息[11-13],使得对于土壤古菌生物多样性的研究仍然匮乏,有关其演替规律仍不明了。近年来兴起的现代分子生物学技术如高通量测序技术等[14-16],为深入探究土壤古菌群落组成、功能及其适应机制提供了良好的契机,有助于阐述古菌群落结构对秸秆还田的响应规律。
本文拟选取江苏省两种长期稻麦轮作田块作为研究对象,利用Illumina测序技术,研究淹水培养期间不同量小麦秸秆施用于两种类型稻田土壤后古菌群落的组成,旨在揭示稻田土壤古菌群落对秸秆还田的响应规律,并结合土壤性质进一步揭示土壤古菌群落迁移的驱动因子。
1 材料与方法 1.1 试验方案供试土壤采自江苏省两种长期稻麦轮作田块,即泰州市的高砂土(GS)和无锡市的黄泥土(HN),土壤风干过2 mm筛,混合均匀后贮存于室温备用,其基本性质见表 1。供试秸秆是成熟收割后的小麦秸秆,来自于江苏省农业科学院小麦试验基地。秸秆晒干后磨碎,过10目筛贮存于4 ℃备用,其中秸秆的含碳量为46.5%,含氮量为0.48%,碳氮比为96.9。
|
|
表 1 两种类型土壤基本性质 Table 1 Basic properties of two paddy soils used in this study |
称取400 g土壤(干质量)于广口瓶中(直径10 cm ×高15 cm),试验设置4种处理,分别为不添加小麦秸秆即对照(Control)、施用10 g·kg-1小麦秸秆(WS 1%)、施用20 g·kg-1小麦秸秆(WS 2%)、施用50 g·kg-1小麦秸秆(WS 5%)。小麦秸秆与土壤混合均匀后,向土壤中添加去离子水使其处于淹水状态,每个处理设置12个重复。将培养瓶置于25 ℃恒温培养箱,避光培养;在淹水培养期间的第0、15、30 d和60 d,分别收集每个处理的3个重复样品的孔隙水和土壤。具体采样过程为:利用土壤孔隙水采样器收集土壤孔隙水,用于溶解性有机碳(DOC)、还原性亚铁[Fe(Ⅱ)]和总铁(Fe)的测定;破坏性采样收集土壤样品,部分土壤风干后用于土壤性质(pH、EC、TC、TN)的测定,部分鲜样保存于-20 ℃冰箱用于古菌群落结构分析。
土壤pH值采用酸度计(pHs-3C型精密pH计,上海雷磁仪器厂)测定(土:水=1:5);土壤EC采用电导仪(DDSJ-318型电导率仪,上海雷磁仪器厂)测定;土壤TC与TN含量采用元素分析仪(Vario MAX CNS,Elementar,Germany)测定,并计算碳氮比值(C/N);孔隙水DOC含量采用有机碳分析仪(Shimadzu TOC - Vcph,Japan)测定;孔隙水Fe(Ⅱ)和总Fe采用邻菲啰啉比色法测定。
1.2 土壤微生物总DNA的提取及其古菌群落分析根据FastDNA® Spin Kit for Soil试剂盒说明书提取土壤中DNA,储存于-20 ℃备用。古菌PCR扩增采用具有barcode的16S rRNA通用引物:519F(5′ - CAGCCGCCGCGGTAA-3′)和806R(5′ - GGACTACNSGGGTMTCTAAT-3′)。20 mL的扩增反应体系包括:10 ng模板DNA、4 mL FastPfu Buffer(5×)、2 mL BSA(2.5 mmol·L-1)、0.8 mL 519F(5 mmol·L-1)、0.8 mL 806R(5 mmol·L-1)、18 mL灭菌水。每个样品包括4个重复体系。扩增条件如下:95 ℃预变性5 min;40个扩增循环,每个循环包括95 ℃变性30 s、60 ℃退火30 s、72 ℃延伸1 min;最后在72 ℃再延伸5 min。混合同一样品的4个PCR产物重复样品,切胶回收并纯化。将PCR产物用蓝色荧光定量系统进行检测定量,之后按照每个样本的测序量要求,进行相应比例的混合。所获得的DNA送到上海凌恩生物有限公司,用于Illumina Miseq测序。
1.3 统计分析所得数据分析采用Excel 2007和SPSS 23.0软件进行处理与统计分析,古菌群落测序数据分析具体请参照文献[17]的方法,古菌群落系统发育树利用I-Sanger云平台进行分析,图形绘制采用SigmaPlot 10.0。
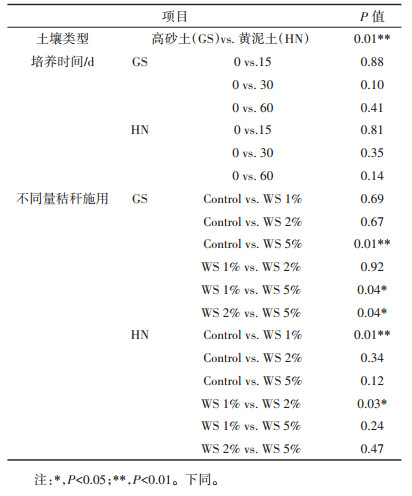
2 结果与分析 2.1 土壤理化性质淹水培养期间,不同量小麦秸秆(0、1%、2%和5%)施用下两种类型土壤的部分理化性质的动态变化见图 1。从图 1可以看出,未施用秸秆时,整个淹水培养期间两种类型水稻土壤的TC、TN、C/N、DOC、Fe(Ⅱ)和Fe浓度整体上并无显著变化。而土壤EC在淹水初期逐渐降低,例如,在淹水的0~15 d期间,高砂土EC从132 mS·cm-1降低至43 mS·cm-1,而黄泥土EC从61.7 mS·cm-1降低至27.5 mS·cm-1;在淹水培养的15~60 d期间,两种类型土壤的EC保持稳定。另外,研究发现两种类型的土壤pH在未施用秸秆时呈现不同的动态变化规律:高砂土壤pH在整个淹水培养期间无显著变化,而黄泥土pH从最初的6.38逐渐降低为6.14,后又逐渐升高为6.77和6.59。以上研究结果表明,0~60 d淹水培养对两种类型土壤的TC、TN、C/N和孔隙水中DOC、Fe(Ⅱ)和总Fe的浓度并无显著影响,但显著降低了黄泥土壤的pH值和两种类型土壤的EC。
|
图 1 0~60 d淹水培养期间不同量秸秆施用下两种类型土壤性质的动态变化 Figure 1 Temporal changes of soil properties during a 60 d incubation period for two paddy soils |
对于施用秸秆的土壤,在整个淹水培养期间,两种类型土壤的TC和TN是逐渐增高的,而土壤C/N及孔隙水DOC、Fe(Ⅱ)和总Fe浓度在淹水培养初期(0~ 15 d或者0~30 d)是逐渐增大的,而在淹水培养后期逐渐降低。当秸秆施用量为5%时,两种类型土壤EC变化趋势存在着显著的差异:黄泥土EC是先升高后降低的,而对于高砂土,在整个厌氧培养期间,EC是从132.4 mS·cm-1逐渐增大到232.3 mS·cm-1。然而当秸秆施用量为1%和2%,在整个厌氧培养期间,两种类型土壤EC都未有显著变化。另外,研究发现施用秸秆的两种类型土壤的pH值呈现不同的动态变化,高砂土壤pH在整个淹水培养期间并无显著变化;而黄泥土pH先有所降低,后又逐渐升高。以上研究结果表明,当添加新鲜有机质时,淹水培养显著增加了土壤TC、TN、C/N、DOC、Fe(Ⅱ)和Fe,而土壤pH和EC的变化与土壤类型密切相关。
与未施用秸秆相比,随着秸秆还田量的增大,土壤pH值逐渐降低,而土壤EC、TC、TN、C/N、DOC、Fe(Ⅱ)和Fe逐渐增加,并且当秸秆还田量为5%时,上述土壤指标改变量最大。以上结果表明,秸秆还田显著降低了土壤pH值,而显著提高了土壤EC、TC、TN、C/N、DOC、Fe(Ⅱ)和Fe含量,并且随着秸秆还田量的增加其改变量逐渐增大。
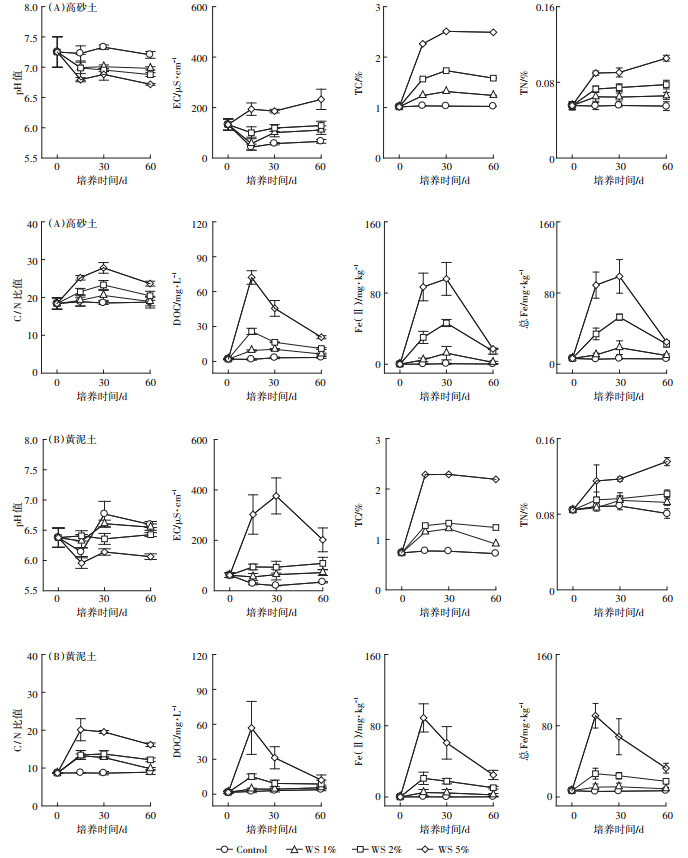
2.2 土壤古菌群落组成利用Illumina高通量测序技术,对78个不同量秸秆施用0、15、30 d和60 d后的土壤样品按照97%相似性对非重复序列(不含单序列)进行OTU聚类。不同量秸秆施用下,淹水培养第0、15、30 d和60 d土壤的古菌群落系统发育树见图 2。
|
图 2 淹水培养期间不同量秸秆施用下两种类型土壤古菌群落的系统发育树 Figure 2 Phylogenetic tree of archaeal community composition in two soils during flooding incubation with different straw additions |
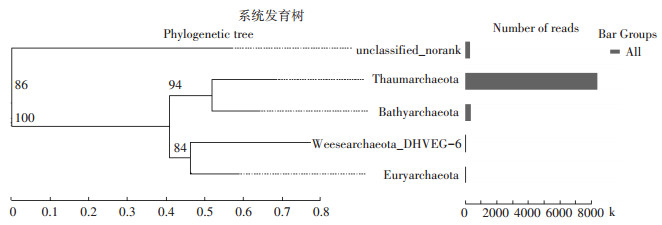
可以看出,未施用秸秆时,高砂土和黄泥土的优势古菌均为奇古菌门(Thaumarchaeota)(图 2),其相对丰度为97.3%和98.1%(图 3);不同秸秆处理下,对奇古菌门相对丰度的显著性差异分析发现,淹水培养第15 d和30 d,秸秆施用对土壤奇古菌门的相对丰度无显著影响;但淹水培养第60 d时,与未施用秸秆相比,秸秆施用下,两种类型稻田土壤中奇古菌门的相对丰度显著降低,并且随着秸秆施用量的增加,奇古菌门的丰度也逐渐降低(P < 0.05)(图 3)。以上结果表明,淹水培养初期,秸秆还田对两种类型稻田土壤中优势古菌(奇古菌门)的丰度并无显著性影响,而淹水培养后期,秸秆还田显著降低了土壤中奇古菌门的丰度,并且秸秆还田量的增加明显抑制了土壤中奇古菌门的丰度。
|
图 3 淹水培养期间不同量秸秆施用下两种类型土壤奇古菌门(Thaumarchaeota)的相对丰度 Figure 3 Relative abundances of Thaumarchaeota in two soils during flooding incubation with different straw additions |
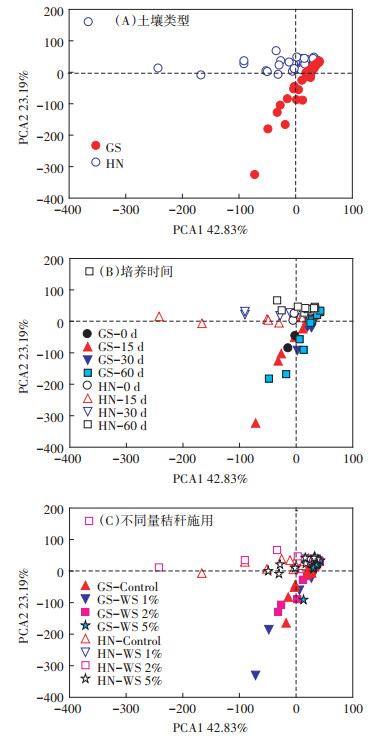
为了进一步探究秸秆还田对土壤中古菌群落组成的影响,我们对不同量秸秆还田下土壤中古菌群落组成进行了主成分分析(PCA)(图 4)。结果显示,两种不同类型的土壤之间古菌群落存在着显著差异(图 4A,表 2),表明土壤类型是土壤微生物群落结构的重要控制因子。进一步分析发现,与未淹水培养的土壤(第0 d)相比,淹水培养(第15、30 d和60 d)期间两种类型土壤古菌群落并无显著变化(图 4B,表 2)。以上研究结果表明,淹水培养时间对两种类型土壤古菌群落结构并无显著性影响。
|
图 4 淹水培养期间不同量秸秆施用下两种类型稻田土壤古菌群落组成的主成分分析 Figure 4 Principle component analysis (PCA) of archaea community composition in two types of soils with various treatments |
|
|
表 2 不同量秸秆施用下两种类型土壤古菌群落结构的差异性分析 Table 2 Significant differences (P value) in archaeal community composition in two soils between different treatments |
与未施用秸秆的土壤相比,秸秆施用量为1%和2%时,并未显著改变高砂土古菌群落结构,而当秸秆施用量为5%时,显著改变了其古菌群落结构;与高砂土不同,当秸秆施用量为1%时,显著改变了黄泥土古菌群落结构,而当秸秆施用量为2%和5%时,并未显著改变其古菌群落结构(图 4C,表 2)。另外,不同量秸秆施用下两种类型土壤古菌群落演替也显著不同。秸秆施用量为5%时,高砂土古菌群落显著不同于秸秆施用量为1%和2%的土壤,而秸秆施用量为1%和2%之间的高砂土古菌群落并无显著差异;然而,对于黄泥土,仅秸秆施用量为1%和2%之间的土壤古菌群落存在着显著性差异(图 4C;表 2)。以上研究结果表明,秸秆还田能够改变两种类型土壤古菌群落结构,而两种类型土壤古菌群落结构对不同量秸秆还田的响应是显著不同的。
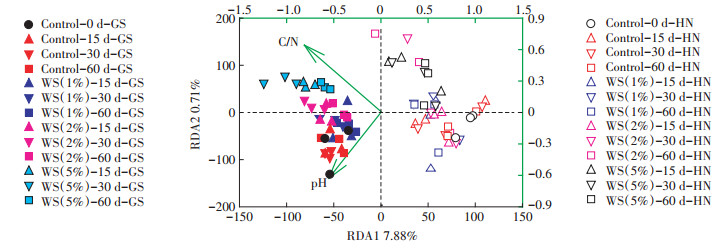
2.3 土壤性质对古菌群落的影响为了进一步探究土壤中古菌群落迁移的主要驱动因子,本文分析了土壤性质与优势古菌(奇古菌门)丰度间的相关性,发现土壤pH、TN和C/N与奇古菌门呈现显著的相关性(表 3)。结果表明pH、TN和C/N是影响奇古菌门差异性的重要因子。进一步通过冗余分析(RDA)对环境因子和土壤古菌群落结构的关系进行探讨,发现在所用的环境因子[pH、EC、TC、TN、C/N、DOC、Fe(Ⅱ)、Fe]中,土壤pH和C/N是影响秸秆还田下两种类型土壤古菌群落迁移的最重要因子(图 5)。以上研究结果表明,秸秆还田很可能主要是通过改变土壤性质来改变优势菌的丰度,进而调控古菌群落结构。
|
|
表 3 奇古菌门(Thaumarchaeota)与土壤性质的相关性分析 Table 3 Spearson correlation coefficient (r) between soil characteristics and the relative abundance of Thaumarchaeota |
|
左下轴代表物种多样性变量值,右上轴代表环境因子变量值 Left and bottom coordinate axis showed species variables values; right and top coordinate axis showed the arrows of environmental variables values 图 5 淹水培养期间不同量秸秆施用下两种类型稻田土壤古菌群落组成的冗余分析 Figure 5 Redundancy analysis (RDA) of archaeal community composition at genus level in two types of soils during flooding incubation with different amounts of wheat straw addition |
古菌是目前生物地球化学研究的热点之一,不仅能在高温、强酸/碱性、高盐、缺氧等极端条件下存在,而且能在普通海洋、土壤等环境介质中存活,其含量巨大,在全球的碳氮元素生物地球化学过程中的作用不可忽视[18]。本研究发现两种类型稻田土壤中主要的优势古菌均为奇古菌门,其主要的属是Candidatus Nitrososphaera,这类古菌已经被报道能够参与氨氧化作用[19]。而早年通过同位素示踪技术对古菌群落组成的分析发现,泉古菌是古菌群落的重要组成,其能利用14C-无机碳和H213CO3进行自养[20-21]。随后,Konneke等从海洋中成功分离培养到第一株奇古菌Nitrosopumilus maritimus,证实了这类奇古菌通过催化氨氧化获取能量进行自养生长的代谢特征[22],因此这类古菌也被称为氨氧化古菌(Ammonia-oxidizing Archaea,AOA)。近年来研究还发现其他一些奇古菌如海绵共生古菌(Cenarchaeum symbiosum)、海洋亚硝化短小古菌(Nitrosopumilus maritimus)、加尔加亚硝化球菌(Nitrososphaera gargensis)、黄石亚硝化热泉菌(Nitrosocaldus yellowstonii)、维也纳亚硝化球菌(Nitrososphaera viennensis EN76)、阿伯丁土壤亚硝化细杆菌(Nitrosotalea devanaterra)等都是重要的氨氧化古菌[22-26]。氨氧化古菌的发现,不仅改变了近百年来人们对氨氧化过程主要由细菌驱动的认识,也为揭示奇古菌门的生理代谢特征及其在自然界物质转化,尤其是氮循环中的作用开启了新的篇章。
大量研究显示土壤性质是调控土壤微生物群落结构的重要因子[27]。已有研究表明土壤pH值是引起微生物群落迁移的重要调控因子[28]。我们研究发现随着秸秆施用量的增大,土壤pH显著降低,这很可能是由于秸秆还田过程中会腐烂分解产生甲酸、乙酸、酚酸等有机酸[29]。进一步的分析也表明pH显著改变了稻田土壤古菌群落组成(图 5)。同时,由于秸秆中含有丰富的碳氮等营养元素,秸秆还田将秸秆中的一些营养元素归还土壤,在一定程度上可以显著增加土壤中碳氮等养分含量[30-32]。本研究中秸秆还田显著提高了土壤碳氮含量及其碳氮比,为微生物的生长提供碳源和氮源,进而改变古菌群落结构。
本研究中两种类型土壤的古菌群落组成对不同量秸秆还田响应的差异很可能是由于不同类型土壤中微生物可利用的基质含量和种类各不相同所导致的。已有研究表明土壤微生物群落的迁移与pH和C/N密切相关(图 5)。本研究中,黄泥土属于偏酸性土壤(pH < 7.0),比较适合古菌优势菌如氨氧化菌的生长[33],同时低秸秆量施用下能够明显提高土壤的C/N比值,为微生物提供碳源和氮源[34],因此显著改变了古菌群落结构;而微生物对土壤中C、N的利用有一定的比例范围,一旦C/N比值变大,势必引起微生物对N源的争夺利用,当秸秆施用量很大时很可能会造成土壤氮缺乏[35],从而降低氨氧化古菌的相对丰度,因此高秸秆施用于酸性土壤后,尽管pH的降低更有利于古菌优势菌如氨氧化古菌的生长,然而过高的C/N反而降低氨氧化古菌的相对丰度。由于pH和C/N对氨氧化古菌的相反效应,因此高秸秆量施用很可能并未引起黄泥土中微生物群落结构的显著性迁移。而相对碱性高砂土(pH > 7.0),pH对微生物菌落的影响作用可能较小,因此高秸秆施用下,C/N对微生物作用更强,从而较高秸秆施用下高砂土古菌群落结构发生显著迁移。
4 结论(1)两种类型稻田土壤的优势古菌均为奇古菌门(Thaumarchaeota)。
(2)两种类型土壤古菌群落组成对不同量秸秆还田响应存在着明显差异。与未施用秸秆的土壤相比,高秸秆施用量(5%)显著改变了高砂土古菌群落组成,而低秸秆施用量(1%)显著改变了黄泥土古菌群落组成。高秸秆施用量(5%)下高砂土古菌群落显著不同于较低秸秆施用量(1%和2%)的土壤,而较低秸秆施用量(1%和2%)之间的土壤古菌群落并无显著差异;而黄泥土仅较低秸秆施用量(1%和2%)之间的土壤古菌群落存在着显著差异。
(3)pH和C/N是秸秆还田下两种类型土壤古菌群落迁移的重要驱动因子。
| [1] |
Wang W, Lai D Y F, Wang C, et al. Effects of rice straw incorporation on active soil organic carbon pools in a subtropical paddy field[J]. Soil & Tillage Research, 2015, 152: 8-16. |
| [2] |
Becker M, Asch F, Maskey S L, et al. Effects of transition season management on soil N dynamics and system N balances in rice-wheat rotations of Nepal[J]. Field Crops Research, 2007, 103(2): 98-108. DOI:10.1016/j.fcr.2007.05.002 |
| [3] |
Khalil M I, Inubushi K. Possibilities to reduce rice straw-induced global warming potential of a sandy paddy soil by combining hydrological manipulations and urea-N fertilizations[J]. Soil Biology and Biochemistry, 2007, 39(10): 2675-2681. DOI:10.1016/j.soilbio.2007.05.003 |
| [4] |
Zou J W, Huang Y, Jiang J Y, et al. A 3-year field measurement of methane and nitrous oxide emissions from rice paddies in China:Effects of water regime, crop residue, and fertilizer application[J]. Global Biogeochemical Cycles, 2005, 19(2): 153-174. |
| [5] |
郭瑞华, 靳红梅, 常志州, 等. 秸秆还田模式对土壤有机碳及腐植酸含量的影响[J]. 农业环境科学学报, 2017, 36(4): 727-733. GUO Rui-hua, JIN Hong-mei, CHANG Zhi-zhou, et al. Effects of returning patterns of straw to field on soil organic carbon and soil humus composition in rice-wheat double cropping systems[J]. Journal of AgroEnvironment Science, 2017, 36(4): 727-733. |
| [6] |
Chen L, Zhang J, Zhao B, et al. Effects of straw amendment and moisture on microbial communities in Chinese fluvo-aquic soil[J]. Journal of Soils & Sediments, 2014, 14(11): 1829-1840. |
| [7] |
赵勇, 李武, 周志华, 等. 秸秆还田后土壤微生物群落结构变化的初步研究[J]. 农业环境科学学报, 2005, 24(6): 1114-1118. ZHAO Yong, LI Wu, ZHOU Zhi-hua, et al. Change of microbial community in straw amended soil[J]. Journal of Agro-Environment Science, 2005, 24(6): 1114-1118. DOI:10.3321/j.issn:1672-2043.2005.06.015 |
| [8] |
于少兰, 乔延路, 韩彦琼, 等. 好氧氨氧化微生物系统发育及生理生态学差异[J]. 微生物学通报, 2015, 42(12): 2457-2465. YU Shao-lan, QIAO Yan-lu, HAN Yan-qiong, et al. Differences between ammonia-oxidizing microorganisms in phylogeny and physiological ecology[J]. Microbiology China, 2015, 42(12): 2457-2465. |
| [9] |
Ouyang Y, Norton J M, Stark J M, et al. Ammonia-oxidizing bacteria are more responsive than archaea to nitrogen source in an agricultural soil[J]. Soil Biology & Biochemistry, 2016, 96: 4-15. |
| [10] |
Thauer R, Kaster A, Seedorf H W, et al. Methanogenic archaea:Ecologically relevant differences in energy conservation[J]. Nature Reviews Microbiology, 2008, 6(8): 579-591. DOI:10.1038/nrmicro1931 |
| [11] |
Chen L, Zhang J, Zhao B, et al. Effects of straw amendment and moisture on microbial communities in Chinese fluvo-aquic soil[J]. Journal of Soils & Sediments, 2014, 14(11): 1829-1840. |
| [12] |
Henriksen T M, Breland T A. Carbon mineralization, fungal and bacterial growth, and enzyme activities as affected by contact between crop residues and soil[J]. Biology & Fertility of Soils, 2002, 35(1): 41-48. |
| [13] |
Zhao F, Xu K. Efficiency of DNA extraction methods on the evaluation of soil microeukaryotic diversity[J]. Acta Ecologica Sinica, 2012, 32(4): 209-214. DOI:10.1016/j.chnaes.2012.05.003 |
| [14] |
Wang N, Yu J G, Zhao Y H, et al. Straw enhanced CO2 and CH4 but decreased N2O emissions from flooded paddy soils:Changes in microbial community compositions[J]. Atmospheric Environment, 2018, 174: 171-179. DOI:10.1016/j.atmosenv.2017.11.054 |
| [15] |
Song M, Luo C, Jiang L, et al. Identification of benzo[J]. Applied & Environmental Microbiology, 2015, 81(21): 7368-7376. |
| [16] |
Lv B Y, Xing M Y, Yang J Y, et al. Pyrosequencing reveals bacterial community differences in composting and vermicomposting on the stabilization of mixed sewage sludge and cattle dung[J]. Applied Microbiology & Biotechnology, 2015, 99(24): 10703-10712. |
| [17] |
王宁, 于建光, 常志州, 等. 稻田土壤真菌群落多样性和组成对麦秸还田的响应[J]. 土壤, 2017, 6: 1115-1120. WANG Ning, YU Jian-guang, CHANG Zhi-zhou, et al. Responses of fungal community diversity and composition in paddy soils to straw return[J]. Soil, 2017, 6: 1115-1120. |
| [18] |
王峰, 萨仁高娃, 马学恩.深海沉积物古菌的研究进展[C].全国兽医病理学、动物病理生理学学术会议论文集, 2009. WANG Feng. SA Rengaowa, MA Xue-en. Advances in research of marine archaea[C]. National Academic Conference on Veterinary Pathology and Animal Pathophysiology, 2009. http://cpfd.cnki.com.cn/Article/CPFDTOTAL-ZGXJ200907001010.htm |
| [19] |
Spang A, Poehlein A, Offre P, et al. The genome of the ammonia-oxidizing candidatus nitrososphaera gargensis:Insights into metabolic versatility and environmental adaptations[J]. Environmental Microbiology, 2012, 14(12): 3122-3145. DOI:10.1111/emi.2012.14.issue-12 |
| [20] |
Pearson A, McNichol A P, Benitez-Nelson B C, et al. Origins of lipid biomarkers in Santa Monica Basin surface sediment:A case study using compound-specific Delta C-14 analysis[J]. Geochimica Et Cosmochimica Acta, 2001, 65(18): 3123-3137. DOI:10.1016/S0016-7037(01)00657-3 |
| [21] |
Wuchter C, Schouten S, Boschker H T S, et al. Bicarbonate uptake by marine Crenarchaeota[J]. EMS Microbiology Letters, 2003, 219(2): 203-207. DOI:10.1016/S0378-1097(03)00060-0 |
| [22] |
Konneke M, Bernhard A E, de la Torre J R, et al. Isolation of an autotrophic ammonia-oxidizing marine archaeon[J]. Nature, 2005, 437(7058): 543-546. DOI:10.1038/nature03911 |
| [23] |
Preston C M, Wu K Y, Molinski T F, et al. A psychrophilic crenarchaeon inhabits a marine sponge:Cenarchaeum symbiosum gen nov, sp, nov[J]. Proceedings of the National Academy of Sciences of the United States of America, 1996, 93(13): 6241-6246. DOI:10.1073/pnas.93.13.6241 |
| [24] |
Hatzenpichler R, Lebecleva E V, Spieck E, et al. A moderately hermophilic ammonia-oxidizing crenarchaeote from a hot spring[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(6): 2134-2139. DOI:10.1073/pnas.0708857105 |
| [25] |
De la Torre J R, Walker C B, Ingalls A E, et al. Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol[J]. Environmental Microbiology, 2008, 10(3): 810-818. DOI:10.1111/emi.2008.10.issue-3 |
| [26] |
Tourna M, Stieglmeier M, Spang A, et al. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(20): 8420-8425. DOI:10.1073/pnas.1013488108 |
| [27] |
Wang N, Ding L J, Xu H J, et al. Variability in responses of bacterial communities and nitrogen oxide emission to urea fertilization among various flooded paddy soils[J]. FEMS Microbiology Ecology, 2015, 91(3): 1-9. |
| [28] |
Shen C, Xiong J, Zhang H, et al. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain[J]. Soil Biology and Biochemistry, 2013, 57: 204-211. DOI:10.1016/j.soilbio.2012.07.013 |
| [29] |
闫洪亮, 王胜楠, 邹洪涛, 等. 秸秆深还田两年对东北半干旱区土壤有机质、pH值及微团聚体的影响[J]. 水土保持研究, 2013, 20(4): 44-48. YAN Hong-liang, WANG Sheng-nan, ZOU Hong-tao, et al. Effects of two years of stalk deeply return to the field on soil microaggregates and soil organic matter and pH value after in semiarid region of the northeastern China[J]. Research of Soil and Water Conservation, 2013, 20(4): 44-48. |
| [30] |
Wang W, Lai D Y F, Wang C, et al. Effects of rice straw incorporation on active soil organic carbon pools in a subtropical paddy field[J]. Soil & Tillage Research, 2015, 152: 8-16. |
| [31] |
Li F, Wang Z, Dai J, et al. Fate of nitrogen from green manure, straw, and fertilizer applied to wheat under different summer fallow management strategies in dryland[J]. Biology & Fertility of Soils, 2015, 51(7): 1-12. |
| [32] |
张丽, 张磊, 鲁剑巍, 等. 添加尿素和秸秆对三熟制水旱轮作土壤各形态氮素的影响[J]. 土壤, 2017, 49(1): 13-18. ZHANG Li, ZHANG Lei, LU Jian-wei, et al. Effects of urea and straw on soil different nitrogen forms under paddy-upland rotation of triple cropping system[J]. Soil, 2017, 49(1): 13-18. DOI:10.3969/j.issn.1673-3908.2017.01.004 |
| [33] |
Hu H W, Xu Z H, He J Z. Ammonia-oxidizing archaea play a predominant role in acid soil nitrification:Advances in agronomy[J]. Elsevier Science & Technology, 2014, 125: 261-302. |
| [34] |
蒋静艳, 黄耀, 宗良纲. 水分管理与秸秆施用对稻田CH4和N2O排放的影响[J]. 中国环境科学, 2003, 23(5): 552-556. JIANG Jing-yan, HUANG Yao, ZONG Liang-gang. Influence of water controlling and straw application on CH4 and N2O emissions from rice field[J]. China Environmental Science, 2003, 23(5): 552-556. DOI:10.3321/j.issn:1000-6923.2003.05.023 |
| [35] |
Shindo H, Nishio T. Immobilization and remineralization of N following addition of wheat straw into soil:Determination of gross N transformation rates by 15N-ammonium isotope dilution technique[J]. Soil Biology and Biochemistry, 2005, 37(3): 425-443. DOI:10.1016/j.soilbio.2004.07.027 |
 2019, Vol. 38
2019, Vol. 38