20世纪以来,氯氟烃和氮氧化物的大量排放导致臭氧层破坏,造成地表紫外辐射B(UV-B)增强。作为重要温室气体之一,N2O大量排放主要源于农业生态系统[1],每年大气中N2O有80%~90%来源于土壤[2],我国稻田每年N2O排放量约为169 Gg N[3],其排放量之大不容忽视。
农业生产中大量施氮造成全球气候变化等生态环境问题[4]。土壤作为陆地氮库,其氮循环转化直接关系到陆地生态系统的物质循环及能量流动[5]。土壤中氮素部分以有机氮的形态存在于土壤中,只有在微生物及土壤酶(脲酶及蛋白酶)作用下将其矿化才能被植物吸收利用[6]。土壤脲酶活性受总氮影响[7],且与碱解氮含量呈显著正相关[8],亚硝酸还原酶活性与土壤NH4+含量存在极显著的线性相关关系[9]。这说明土壤酶活性对氮素转化起着重要的作用。在低矿物质氮含量中,蛋白酶活性会被NH4+抑制[10]。土壤中有效氮以铵态氮(NH4+-N)和硝态氮(NO3--N)为主要形式存在,其变化情况与土壤氮素迁移转化有重要关系[11]。N2O排放与土壤中NO3--N含量显著相关[12-14],与NH4+-N含量也显著相关[9]。因此了解土壤中氮素转化对研究N2O的排放有积极作用。
紫外辐射(Ultraviolet radiation)是指波长范围在0~400 nm的光辐射。根据其波段不同可分为UV-A、UV-B、UV-C[15]。UV-A辐射基本能全部到达地表,但通常情况下UV-A辐射对地表动植物伤害非常小;UV-C极易被大气层中O3吸收,能够到达地表的辐射量基本可忽略不计。而UV-B辐射能够被O3部分吸收,但到达地面后会对地表动植物造成影响,在紫外辐射所有波段中,B波段对地表生物影响最大。目前南极上空的臭氧层空洞已经开始修复,2015年10月创纪录的月平均臭氧空洞面积为2530万km2[16]。但人造二氯甲烷排放量迅速增长,延缓臭氧层的修复[17];含氯溴的短暂存在物质消耗平流层下层的臭氧[18],臭氧衰减导致的地表UV-B辐射增强仍不可忽视。UV-B辐射增强直接或间接影响土壤中氮的转化,会促进无机态氮转化,尤其促进硝态氮(NO3--N)向铵态氮(NH4+-N)转化[19],导致NH4+-N含量显著增加[20]。累积净矿化氮量也随辐射强度变化而变化,表现为矿质氮累积[21]。UV-B辐射增强显著影响根际氨氧化细菌数量及土壤酶活性,如抑制脲酶活性等[22];还影响植株代谢,进而通过根系分泌物影响根际土壤微生物,最终影响N2O等温室气体的排放[23]。UV-B辐射增强提高稻田N2O排放通量[24],却降低了冬小麦[25]、大豆[26]的N2O累积排放量。然而,其影响农田土壤氮素转化与N2O排放的过程及机理研究有待进一步加强。
元阳梯田地处云南高原,是我国西南地区典型的冬水田。当地UV-B辐射较强,稻田终年淹水。已有较多关于温室气体排放及碳转化的研究在该地开展,研究结果主要有:UV-B辐射增强改变水稻根系低分子量有机酸的分泌量[27],促进当地稻田CH4排放[28],且会导致水稻各部位N含量显著增加[29]。本文以元阳梯田地方水稻品种为研究对象,开展大田试验,在水稻生长期进行不同强度(0、2.5、5.0 kJ·m-2和7.5 kJ·m-2)UV-B辐射处理,研究UV-B辐射增强对稻田土壤氮转化相关酶活性、无机氮含量和N2O排放通量的影响,探讨UV-B辐射增强条件下氮转化机理,以期为了解农田系统土壤氮转化提供基础数据和科学依据。
1 材料与方法 1.1 试验地概况试验地位于云南省元阳县新街镇箐口村(23°07′ N,102°44′ E),该梯田海拔高度为1600 m,年平均气温为15 ℃,年均降水量为1 397.6 mm。供试土壤理化性质如下:pH为5.32,有机质含量为26.8 g·kg-1,全氮、全磷、全钾含量分别为1.91、0.65 g·kg-1和16.43 g·kg-1,碱解氮、速效磷、速效钾含量分别为76.44、15.76 mg·kg-1和101 mg·kg-1。
1.2 试验设计试验水稻品种为白脚老粳,是元阳梯田地方水稻品种。在2018年3月18播种育苗,5月9日移栽到试验小区。试验设置4个处理水平,每个处理设置3个重复。每个小区设计为长3.9 m、宽2.25 m,小区周边6行和4列水稻被设为保护行。在水稻生长期不使用农药和化肥,且一直保持淹水状态。
UV-B辐射处理:在每一行水稻正上方悬挂10支40 W UV-B灯管(UV308,光谱为280~320 nm),模拟UV-B辐射增强。用0.13 mm醋酸纤维素膜滤除280 nm以下UV-C波段光线;聚酯薄膜滤除UV-C和UVB辐射,UV-B辐射的生物学效应考虑为UV-B处理组和UV-A对照组下的生物学效应的差值,消除处理组中UV-A对UV-B效应的影响。用紫外辐射测定仪(北京师范大学光电仪器厂)测定波长为297 nm时的辐射强度来确定紫外辐射水平,且根据水稻植株生长来调节灯管高度以控制辐照度(以植株上部计)。设0(自然光)、2.5、5.0 kJ·m-2和7.5 kJ·m-2 4个处理水平,分别相当于元阳梯田0、10%、20%和30%的臭氧衰减(夏至日UV-B辐射背景值为10.0 kJ·m-2)。为保证与其他辐射处理的一致性,0辐射水平同样要悬挂未安装灯管的管架。从水稻秧苗移栽返青后,开始紫外辐射处理至成熟,每日10:00—17:00辐照(阴雨天除外)。
1.3 测定方法在水稻生长的分蘖期、拔节期、孕穗期、成熟期采集土壤样品,采用五点取样法取得稻田表层(0~20 cm)土壤,按四分法去掉多余土壤。新鲜土样初步去除石砾等杂质后装入低温储藏箱运回实验室。
土壤硝酸还原酶的测定:取风干土样加CaCO3、2,4-二硝基酚溶液、KNO3、葡萄糖溶液混匀,30 ℃恒温培养24 h,加入蒸馏水、铝钾矾饱和溶液,振荡后过滤,取滤液加入显色剂用蒸馏水定容,波长520 nm下比色,每个处理土样需做无基质对照[30]。
土壤亚硝酸还原酶的测定:取风干土样加CaCO3、NaNO2、葡萄糖溶液混匀,30 ℃恒温培养24 h,加入蒸馏水、铝钾矾饱和溶液,振荡后过滤,取滤液加入显色剂用蒸馏水定容,波长520 nm下比色,每个处理土样需做无基质对照[31]。
土壤蛋白酶的测定:取风干土样加1%酪素溶液、甲苯,振荡后在30 ℃恒温培养24 h,加入0.1 mol·L-1硫酸及硫酸钠溶液,离心,取上清液2 mL定容至50 mL,进行比色测定[32]。
土壤脲酶的测定:取风干土样加甲苯置于50 mL三角瓶中,静置15 min,加10%尿素液和pH 6.7柠檬酸盐缓冲液,30 ℃恒温培养24 h,过滤后取3 mL滤液于50 mL容量瓶,进行比色测定[32]。
土壤NO3--N的测定(酚二磺酸比色法):取新鲜土样、CaSO4·2H2O和水于三角瓶中,振荡后过滤,取滤液加CaCO3置于瓷蒸发皿,水浴蒸干,加入酚二磺酸试剂,静置后加水、1:1 NH4OH至溶液微黄,定容至100 mL容量瓶,在分光光度计上于波长420 nm处比色[33]。
土壤NH4+-N的测定(KCl-靛酚蓝比色法):取新鲜土样、KCl溶液于150 mL三角瓶中,振荡,过滤,取滤液、苯酚溶液和次氯酸钠碱性溶液放入容量瓶中,摇匀。在20 ℃左右室温下放置1 h后加入掩蔽剂,用水定容。最后将溶液进行比色[33]。
N2O排放通量的测定:采用静态箱-气相色谱法收集田间气体样品[34]。分别于水稻生长的分蘖期、拔节期、孕穗期、成熟期进行采样。采用密封静态箱采集,每次采样时间固定在天气晴朗的9:00—11:00,采样时先将箱体罩于水稻上,用双链球手动将箱内气体泵入到真空铝箔气体袋中,采样时间分别为罩箱后0、10、20 min和30 min。气体样品采用Agilent 7890B气相色谱仪分析。
计算公式:F=ρ×h×dc/dt×273/(273+T)
式中:F为气体排放通量,µg·m-2·h-1;ρ为标准状况下气体密度,1.25 kg·m-3;h为采样箱高度,m;dc/dt为单位时间内气体浓度线性变化率,nL·L-1·h-1;T为箱内温度,K。
1.4 数据处理使用Excel对试验原始数据进行记录整理,用SPSS 23对数据进行数据分析(单因素方差分析和显著性检验),用Origin Pro9.0进行绘图。
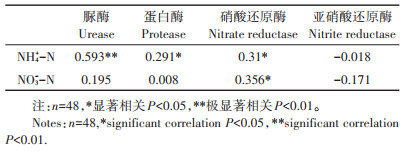
2 结果与分析 2.1 UV-B辐射增强对稻田土壤氮素转化酶活性的影响UV-B辐射增强导致4种土壤氮转化酶在水稻生长的不同时期呈现不同的变化趋势(见图 1)。分蘖期,5.0 kJ·m-2 UV-B辐射增强处理显著增加硝酸还原酶活性;拔节期,3个强度的UV-B辐射增强处理硝酸还原酶活性显著降低,降幅分别为33.33%、34.41%、23.19%。孕穗期,5.0 kJ·m-2和7.5 kJ·m-2 UV-B辐射增强处理下硝酸还原酶活性显著增加。成熟期,UVB辐射增强处理均显著增加硝酸还原酶活性。自然光处理下,4个时期硝酸还原酶活性差异性显著,孕穗期3个强度的UV-B辐射增强处理硝酸还原酶活性均显著高于其他时期。
|
不同大写字母表示同一处理不同时期间差异显著,不同小写字母表示同一时期不同处理间差异显著(P < 0.05)。下同 Different uppercase letters indicate significant differences during different periods of the same treatment, while different lowercase letters indicate significant differences between different treatments during the same period(P < 0.05). The same below 图 1 UV-B辐射对土壤氮转化酶活性的影响 Figure 1 Effect of UV-B radiation on soil nitrogen invertase activity |
分蘖期UV-B辐射增强处理导致亚硝酸还原酶活性显著降低,而UV-B辐射增强处理间无显著差异。在拔节期,5.0 kJ·m-2和7.5 kJ·m-2 UV-B辐射增强处理使亚硝酸还原酶活性显著增加;孕穗期,5.0 kJ·m-2 UV-B辐射增强处理使亚硝酸还原酶活性显著降低,降幅为25.23%。成熟期2.5 kJ·m-2和7.5 kJ·m-2 UV-B辐射增强处理显著降低亚硝酸还原酶活性。自然光处理及其他三个强度的UV-B辐射增强处理下,孕穗期时亚硝酸还原酶活性显著高于其他时期。
UV-B辐射增强处理使蛋白酶活性在拔节期和成熟期显著升高,且在成熟期UV-B辐射增强处理间无显著差异。孕穗期UV-B辐射增强处理使蛋白酶活性显著降低。自然光处理及2.5 kJ·m-2和5.0 kJ·m-2 UV-B辐射增强处理下,分蘖期时蛋白酶活性显著高于其他时期。7.5 kJ·m-2 UV-B辐射增强处理下,孕穗期时蛋白酶活性显著低于其他时期。
脲酶活性在分蘖期UV-B辐射增强处理下显著降低,降幅范围为14.92%~20.52%。拔节期和孕穗期,UV-B辐射增强处理使脲酶活性显著降低。成熟期5.0 kJ·m-2 UV-B辐射增强处理显著增加脲酶活性。自然光处理及3个强度的UV-B辐射增强处理下,分蘖期时脲酶活性显著高于其他时期。
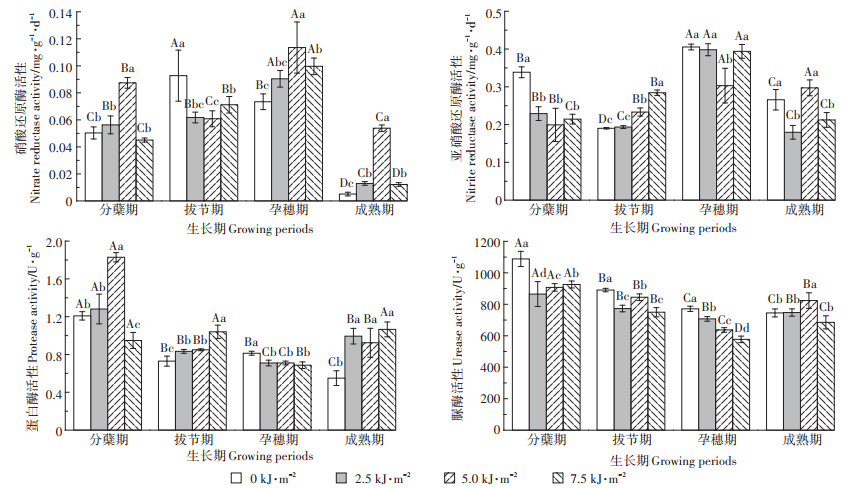
2.2 UV-B辐射增强对稻田土壤无机氮含量的影响由图 2可知,分蘖期,5.0 kJ·m-2 UV-B辐射增强处理土壤NO3--N含量显著增加,2.5 kJ·m-2 UV-B辐射增强处理显著降低土壤NO3--N含量。拔节期,5.0 kJ·m-2和7.5 kJ·m-2 UV-B辐射增强处理显著增加NO3--N含量。孕穗期,2.5 kJ·m-2 UV-B辐射增强处理NO3--N含量增加了1.72 mg·kg-1,7.5 kJ·m-2 UV-B辐射增强处理降低了49%。成熟期,7.5 kJ·m-2 UV-B辐射增强处理NO3--N含量变化幅度最大,增幅为91%。自然光处理及3个强度的UV-B辐射增强处理下,孕穗期时NO3--N含量显著高于其他时期。不同生长阶段之间来看,除孕穗期外,UV-B辐射使土壤NO3--N含量的变化趋势基本一致。
|
图 2 UV-B辐射对土壤NO3--N、NH4+-N含量的影响 Figure 2 Effect of UV-B radiation on NO3--N, NH4+-N content in soil |
UV-B辐射增强处理对稻田土壤NH4+-N含量的影响在不同生长期变化趋势不同。UV-B辐射增强处理使分蘖期和孕穗期NH4+-N含量显著降低。拔节期7.5 kJ·m-2 UV-B辐射增强处理显著降低NH4+-N含量。成熟期,UV-B辐射增强处理下NH4+-N含量均显著降低,其中2.5 kJ·m-2 UV-B辐射增强处理降低了11.85 mg·kg-1,而5.0 kJ·m-2 UV-B辐射增强处理降幅最大,为74%。自然光处理及3个强度的UV-B辐射增强处理下,分蘖期时NH4+-N含量显著高于其他时期。
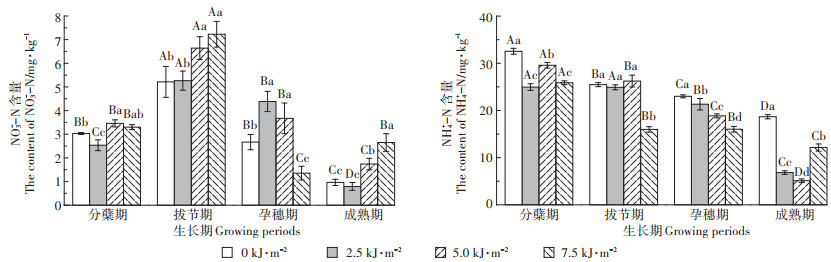
2.3 UV-B辐射增强对稻田N2O排放的影响从图 3可以看出,在水稻拔节期和孕穗期UV-B辐射增强使N2O排放通量显著降低,成熟期N2O排放通量显著增加。拔节期2.5 kJ·m-2 UV-B辐射处理下,N2O排放通量最高为40.76 μg·m-2·h-1。自然光处理和2.5 kJ·m-2 UV-B辐射处理下,拔节期时N2O排放通量显著高于其他时期。5.0 kJ·m-2 UV-B辐射处理下,分蘖期时N2O排放通量显著高于其他时期。7.5 kJ·m-2 UV-B辐射处理下,成熟期时N2O排放通量显著高于其他时期。总体来看,UV-B辐射增强处理下N2O总排放量显著降低。
|
图 3 UV-B辐射对N2O排放通量的影响 Figure 3 Effect of UV-B radiation on N2O emission flux |
由表 1可知,NH4+-N含量与脲酶活性呈极显著正相关(P < 0.01),与蛋白酶、硝酸还原酶活性呈显著正相关(P < 0.05)。NO3--N含量与硝酸还原酶活性呈显著正相关(P < 0.05)。NO3--N含量与N2O排放通量之间无相关性,从图 4可见,NH4+-N含量与NO3--N含量、N2O排放通量呈极显著正相关(P < 0.01)。
|
|
表 1 土壤NO3--N、NH4+N含量与氮转化酶活性的相关性 Table 1 Correlation between soil NO3--N, NH4+N content and nitrogen invertase activity |
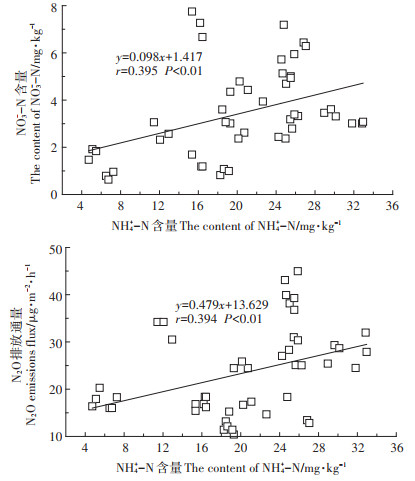
|
图 4 土壤NH4+-N含量与NO3--N含量、N2O排放通量的相关性 Figure 4 Correlation between NH4+-N content and NO3--N content and N2O emission flux in soil |
本研究中UV-B辐射处理下NH4+-N含量显著降低。分析水稻生育期土壤酶活性发现,脲酶活性在UV-B辐射处理后的变化与NH4+-N变化基本一致,两者呈极显著正相关。这是因为脲酶、蛋白酶的最终产物都是NH4+-N,酶活性越强,产物越多[35]。同时试验还发现UV-B辐射使土壤中NO3--N含量显著增加,NH4+-N含量显著降低,可能是土壤有机质在矿化过程中产生无机氮,其中以NO3--N为主,少量的NH4+-N也会在UV-B辐射的促进下部分转化为NO3--N [5]。NH4+-N含量增加有利于分蘖盛期前水稻对氮的吸收[36],因此分蘖期NH4+-N含量逐渐降低。NO3--N则在前期累积,后期被水稻吸收利用,逐渐降低。
UV-B辐射无法直接穿透水层作用于土壤酶,是通过影响植株,改变土壤根际环境进而影响土壤酶活性。有研究发现UV-B辐射增强会导致根际土壤亚硝酸细菌数量增加[37],这也解释了本试验中土壤硝化作用增强的原因。因此随着UV-B辐射增强NH4+大量转化为NO3-,最终导致土壤中NH4+-N含量减少,NO3--N含量的增加。拔节期时土壤脲酶活性升高,可能是气温升高使得作物根系活动活跃,使脲酶活性增强;在水稻孕穗期及成熟期脲酶保持较高的活性,这是由于该阶段水稻生长需吸收更多氮素,而土壤脲酶活性增强促进有机氮素转化为速效氮,为植物提供更多氮素[38]。整体来看,UV-B辐射增强使土壤脲酶活性降低,这与张令瑄等[39]的研究结果相同,UV-B辐射对作物的根系生物量产生抑制作用,进而影响根系分泌物的产生,使土壤酶活性降低。另外太阳辐射对脲酶的影响直接且敏感[40]。同样作为水解酶,土壤蛋白酶可以分解土壤中有机含氮化合物,其不仅会限制土壤氮矿化过程,而且还能反映土壤质量状况[41]。土壤中蛋白酶能够与无机化合物结合受到保护,降低其活性[42],还可以与自然界中存在的腐殖质等物质络合[43]。本研究中,孕穗期土壤硝酸还原酶活性较其他时期最高,原因可能与该时期水稻根系大量泌氧使土壤中硝化作用加剧有关[44]。硝酸还原酶是参与反硝化过程的第一个酶,其活性受到底物数量的影响[45]。UV-B辐射增强下土壤NO3--N含量增加,硝酸还原酶活性因而随之增强。通常认为,在嫌气条件下,土壤硝酸还原酶活性更强[46],水稻拔节期时,试验地降水量增加,氧气含量低,而成熟期降雨量减少,硝酸还原酶活性降低。同时陈永勤等[47]研究发现,光照强度增加硝酸还原酶活性显著增加,而亚硝酸还原酶活性无显著变化。有研究表明UV-B辐射对植物存在低促高抑的现象[48],因此可能会对根际环境造成同样的影响,这也解释了本试验中存在的部分现象。
3.2 N2O排放对UV-B辐射的响应稻田土壤无机氮与温室气体的产生关系密切[49],水稻根际环境变化会影响无机氮和温室气体[50-51]。N2O产生的重要因素是底物和微生物,土壤释放的N2O主要是氮素在微生物作用下,经过硝化和反硝化作用产生[52]。UV-B辐射增强影响作物根际分泌物产生,进而导致土壤微生物的种类和活性受到影响[27]。有研究表明,UV-B辐射增强导致土壤细菌数量、真菌数量及放线菌数量下降[53]。紫外辐射下N2O排放通量降低与反硝化细菌数量减少有关。其次,本研究中发现成熟期NO3--N含量随UV-B辐射增强显著增加,而作为N2O产生底物,其含量增加N2O排放量随之增加。稻田淹水状况同样会影响N2O排放,水层较厚时N2O排放降低[54]。拔节期时稻田降水量增加,因此N2O排放量降低,成熟期时降水量降低,N2O排放量有所回升。
N2O排放通量与不同形态氮素及氮转化酶之间存在相关性(图 5)。NO3--N作为硝酸还原酶的底物,其数量增加促进了硝酸还原酶活性升高,以发挥其最大活性[50]。NH4+-N含量与N2O排放通量呈极显著正相关,NH4+-N作为硝化作用的底物,为参与硝化作用和反硝化作用的微生物提供代谢所需的能源,从而使土壤N2O排放通量增加[11]。UV-B辐射通过改变根际土壤中氮转化相关酶活性,进而发生一系列变化,对N2O排放产生影响。
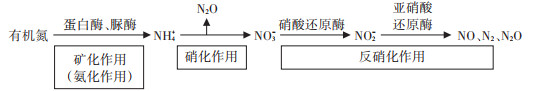
|
图 5 N2O产生途径示意图 Figure 5 Schematic diagram of N2O production pathway |
UV-B辐射增强增加土壤硝酸还原酶和蛋白酶活性,抑制了土壤脲酶、亚硝酸还原酶活性;UV-B辐射增强处理增加水稻生育期稻田土壤中NO3--N含量,显著降低了水稻生育期稻田土壤NH4+-N含量;UV-B辐射通过间接作用提高土壤氮转化相关酶活性,促进土壤氮的转化,降低了拔节期和孕穗期N2O的排放。
| [1] |
蒋静艳, 胡正华, 牛传坡. UV-B辐射增强对小麦秸秆化学成分及其施用后土壤N2O排放的影响[J]. 应用生态学报, 2010, 21(10): 2715-2720. JIANG Jing-yan, HU Zheng-hua, NIU Chuan-po. Effects of elevated ultraviolet-B radiation on the chemical composition of wheat straw and the N2O emission from soil amended with the straw[J]. Chinese Journal of Applied Ecology, 2010, 21(10): 2715-2720. |
| [2] |
Hansen J E, Lacis A A. Sun and dust versus greenhouse gases:An assessment of their relative roles in global climate change[J]. Nature, 1990, 346(6286): 713-719. |
| [3] |
Zou J W, Huang Y, Lu Y Y, et al. Direct emission factor for N2O from rice-winter wheat rotation systems in Southeast China[J]. Atmospheric Environment, 2005, 39(26): 4755-4765. |
| [4] |
Bacon S A, Mau R, Neto F M, et al. Effect of climate warming on maize production in Timor-Leste:Interaction with nitrogen supply[J]. Crop & Pasture Science, 2016, 67(2): 156-166. |
| [5] |
徐鹏, 王秋敏, 蒋梦蝶, 等. UV-B辐射促进红壤水稻土中碳氮转化[J]. 农业环境科学学报, 2017, 36(4): 793-798. XU Peng, WANG Qiu-min, JIANG Meng-die, et al. UV-B radiation facilitates the transformation of carbon and nitrogen in red paddy soils[J]. Journal of Agro-Environment Science, 2017, 36(4): 793-798. |
| [6] |
Antti-Jussi Kieloaho, Mari Pihlatie, Maria Dominguez Carrasco, et al. Stimulation of soil organic nitrogen pool:The effect of plant and soil organic matter degrading enzymes[J]. Soil Biology and Biochemistry, 2016, 96: 97-106. |
| [7] |
Đukić D, Mandić L, Vesković S, et al. Correlation between fertilizer type and rate, urease activity and total nitrogen and ammonium nitrogen contents in a chernozem soil under wheat[J]. Agriculture & Forestry, 2015, 61(3): 43-51. |
| [8] |
闫洪奎, 于泽, 王欣然, 等. 基于旋耕玉米秸秆还田条件下土壤微生物、酶及速效养分的动态特征[J]. 水土保持学报, 2018, 32(2): 276-282. YAN Hong-kui, YU Ze, WANG Xin-ran, et al. Dynamic characteristics of soil microorganisms, enzymes and available nutrients under the conditions of the corn straw returning based on rotary tillage[J]. Journal of Soil and Water Conservation, 2018, 32(2): 276-282. |
| [9] |
陈利军, 武志杰, 姜勇, 等. 与氮转化有关的土壤酶活性对抑制剂施用的响应[J]. 应用生态学报, 2002, 13(9): 1099-1103. CHEN Li-jun, WU Zhi-jie, JIANG Yong, et al. Response of N transformation related soil enzyme activities to inhibitor applications[J]. Chinese Journal of Applied Ecology, 2002, 13(9): 1099-1103. |
| [10] |
Geisseler D, Horwath W R. Regulation of extracellular protease activity in soil in response to different sources and concentrations of nitrogen and carbon[J]. Soil Biology & Biochemistry, 2008, 40(12): 3040-3048. |
| [11] |
马芬, 马红亮, 邱泓, 等. 水分状况与不同形态氮添加对亚热带森林土壤氮素净转化速率及N2O排放的影响[J]. 应用生态学报, 2015, 26(2): 379-387. MA Fen, MA Hong-liang, QIU Hong, et al. Effects of water levels and the additions of different nitrogen forms on soil net nitrogen transformation rate and N2O emission in subtropical forest soils[J]. Chinese Journal of Applied Ecology, 2015, 26(2): 379-387. |
| [12] |
Gu X, Chen K, Fan C. Preliminary evidence of effects of Phragmites australis growth on N2O emissions by laboratory microcosms[J]. Ecological Engineering, 2015, 83: 33-38. |
| [13] |
Jahangir M M R, Johnston P, Barrett M, et al. Denitrification and indirect N2O emissions in groundwater:Hydrologic and biogeochemical influences[J]. Journal of Contaminant Hydrology, 2013, 152(152): 70-81. |
| [14] |
Macdonald J D, Rochette P, Chantigny M H, et al. Ploughing a poorly drained grassland reduced N2O emissions compared to chemical fallow[J]. Soil & Tillage Research, 2011, 111(2): 123-132. |
| [15] |
Misovic M, Milenkovic D, Martinovic T, et al. Short-term exposure to UV-A, UV-B, and UV-C irradiation induces alteration in cytoskeleton and autophagy in human keratinocytes[J]. Ultrastructural Pathology, 2013, 37(4): 241-248. |
| [16] |
Solomon S, Ivy D J, Kinnison D, et al. Emergence of healing in the Antarctic ozone layer[J]. Science, 2016, 353(2696): 2601-2474. |
| [17] |
Hossaini R, Chipperfield M P, Montzka S A, et al. The increasing threat to stratospheric ozone from dichloromethane[J]. Nature Communications, 2017, 8: 15962. |
| [18] |
Hossaini R, Chipperfield M P, Montzka S A, et al. Efficiency of shortlived halogens at influencing climate through depletion of stratospheric ozone[J]. Nature Geoscience, 2015, 8(3): 186-190. |
| [19] |
Speir T W, Kettles H A, More R D. Aerobic emissions of N2O and N2 from soil cores:Measurement procedures using 13N-labelled NO3- and NH4+[J]. Soil Biology and Biochemistry, 1995, 27(10): 1298. |
| [20] |
胡正华, 蒋静艳, 牛传坡, 等. 地表UV-B辐射增强对土壤-大豆系统N2O排放的影响[J]. 生态学报, 2009, 29(12): 6754-6763. HU Zheng-hua, JIANG Jing-yan, NIU Chuan-po, et al. Impacts of enhanced UV-B radiation on N2O emissions from soil-soybean systems[J]. Acta Ecologica Sinica, 2009, 29(12): 6754-6763. |
| [21] |
蒋梦蝶, 王秋敏, 徐鹏, 等. 不同水分梯度下UV-B辐射对2个稻田土壤碳氮转化的影响[J]. 环境科学, 2017, 38(11): 4819-4827. JIANG Meng-die, WANG Qiu-min, XU Peng, et al. Effects of UV-B radiation on soil carbon and nitrogen transformation under different soil moisture contents from two paddy fields[J]. Environmental Science, 2017, 38(11): 4819-4827. |
| [22] |
吕志伟, 万国峰, 张朋, 等. CO2倍增和UV-B辐射增强对大豆根际氨氧化细菌数量及土壤酶活的影响[J]. 大豆科学, 2012, 31(1): 69-72. LÜ Zhi-wei, WAN Guo-feng, ZHANG Peng, et al. Effects of doubled CO2 and enhanced UV-B radiation on rhizosphere ammonia-oxidizing bacteria and soil enzymes in soybean(Glycine max Merr.)[J]. Soybean Science, 2012, 31(1): 69-72. |
| [23] |
王灿, 李虹茹, 湛方栋, 等. UV-B辐射对元阳梯田稻田土壤活性有机碳含量与温室气体排放的影响[J]. 农业环境科学学报, 2018, 37(2): 383-391. WANG Can, LI Hong-ru, ZHAN Fang-dong, et al. Effects of enhanced UV-B radiation on the content of soil active organic carbon and greenhouse gas emission from a rice paddy in Yuanyang Terraces[J]. Journal of Agro-Environment Science, 2018, 37(2): 383-391. |
| [24] |
肇思迪, 娄运生, 张祎玮, 等. UV-B增强下施硅对稻田CH4和N2O排放及其增温潜势的影响[J]. 生态学报, 2017, 37(14): 4715-4724. ZHAO Si-di, LOU Yun-sheng, ZHANG Yi-wei, et al. Effect of silicate supply on CH4 and N2O emissions and their global warming potentials in a Chinese paddy soil under enhanced UV-B radiation[J]. Acta Ecologica Sinica, 2017, 37(14): 4715-4724. |
| [25] |
胡正华, 蒋静艳, 牛传坡, 等. 地表UV-B辐射增强对土壤-冬小麦系统呼吸速率和N2O排放的影响[J]. 环境科学, 2007, 28(3): 449-454. HU Zheng-hua, JIANG Jing-yan, NIU Chuan-po, et al. Influence of enhanced UV-B radiation on respiration rate and N2O emission from soil-winter wheat system[J]. Environmental Science, 2007, 28(3): 449-454. |
| [26] |
胡正华, 杨燕萍, 李涵茂, 等. UV-B增强与秸秆施用对土壤-大豆系统呼吸速率和N2O排放的影响[J]. 中国环境科学, 2010, 30(4): 539-543. HU Zheng-hua, YANG Yan-ping, LI Han-mao, et al. Combined effect of enhanced UV-B radiation and straw addition on respiration rate and N2O emission from soil-soybean system[J]. China Environmental Science, 2010, 30(4): 539-543. |
| [27] |
何永美, 湛方栋, 吴炯, 等. UV-B辐射对元阳梯田水稻根系LMWOAs分泌量和根际微生物数量的影响[J]. 农业环境科学学报, 2016, 35(4): 613-619. HE Yong-mei, ZHAN Fang-dong, WU Jiong, et al. Effects of UV-B radiation on rice roots-exudated LMWOAs and rhizospheric microorganism quantities in a paddy field of Yuanyang Terraces, Yunnan Province[J]. Journal of Agro-Environment Science, 2016, 35(4): 613-619. |
| [28] |
徐渭渭, 何永美, 湛方栋, 等. UV-B辐射增强对元阳哈尼梯田稻田CH4排放规律的影响[J]. 生态学报, 2015, 35(5): 1329-1336. XU Wei-wei, HE Yong-mei, ZHAN Fang-dong, et al. Effect of enhanced UV-B radiation on CH4 emission from paddy field in Yuanyang Hani Terraces[J]. Acta Ecologica Sinica, 2015, 35(5): 1329-1336. |
| [29] |
刘畅, 何永美, 祖艳群, 等. 增强UV-B辐射对元阳梯田2个地方水稻品种N营养累积的影响[J]. 农业环境科学学报, 2013, 32(8): 1493-1499. LIU Chang, HE Yong-mei, ZU Yan-qun, et al. Effects of enhanced UV-B Radiation on N accumulation of two traditional rice colonies in Yuanyang Terraces under field conditions[J]. Journal of Agro-Environment Science, 2013, 32(8): 1493-1499. |
| [30] |
武志杰, 隽英华, 陈利军, 等.一种检测土壤硝酸还原酶活性的分析方法: CN101271060[P]. 2008-09-24. WU Zhi-jie, JUAN Ying-hua, CHEN Li-jun, et al. Analytical method for detecting soil nitrate reductase activity: CN101271060[P]. 2008-09-24. |
| [31] |
武志杰, 孙志梅, 张丽莉.一种检测土壤亚硝酸还原酶活性的分析方法: CN1979134[P]. 2007-06-13. WU Zhi-jie, SUN Zhi-mei, ZHANG Li-li. Analytical method for detecting soil nitrite reductase activity: CN1979134[P]. 2007-06-13. |
| [32] |
关松荫. 土壤酶及其研究方法[M]. 北京: 中国农业出版社, 1986. GUAN Song-yin. Soil enzyme and its research method[M]. Beijing: China Agriculture Press, 1986. |
| [33] |
鲍士旦. 土壤农化分析[M]. 版本. 3版: 中国农业出版社, 2000. BAO Shi-dan. Soil and agricultural chemistry analysis[M]. 3rd Edition. Beijing: China Agriculture Press, 2000. |
| [34] |
娄运生, 周文鳞. UV-B辐射增强对抗除草剂转基因水稻CH4排放的影响[J]. 生态学报, 2012, 32(15): 4731-4736. LOU Yun-sheng, ZHOU Wen-lin. Effect of elevated ultraviolet-B (UV-B)radiation on CH4 emission in herbicide resistant transgenic rice from a paddy soil[J]. Acta Ecologica Sinica, 2012, 32(15): 4731-4736. |
| [35] |
李源, 祝惠, 袁星. 冻融交替对黑土氮素转化及酶活性的影响[J]. 土壤学报, 2014, 51(5): 1103-1109. LI Yuan, ZHU Hui, YUAN Xing. Influence of freezing and thawing cycles on net nitrogen transformation and enzyme activity in black soils[J]. Acta Pedologica Sinica, 2014, 51(5): 1103-1109. |
| [36] |
孙园园, 孙永健, 杨志远, 等. 不同形态氮肥与结实期水分胁迫对水稻氮素利用及产量的影响[J]. 中国生态农业学报, 2013, 21(3): 274-281. SUN Yuan-yuan, SUN Yong-jian, YANG Zhi-yuan, et al. Nitrogen use efficiency and yield of rice under different nitrogen and water stress conditions at grain-filling stage[J]. Chinese Journal of Eco-Agriculture, 2013, 21(3): 274-281. |
| [37] |
李元, 杨济龙, 王勋陵. 紫外辐射增加对春小麦根际土壤微生物种群数量的影响[J]. 中国环境科学, 1999, 19(2): 157-160. LI Yuan, YANG Ji-long, WANG Xun-ling. The effect of UV-B radiation on the population quantity of spring wheat rhizosphere microorganisms[J]. China Environmental Science, 1999, 19(2): 157-160. |
| [38] |
娄运生, 程焕友, 韩艳. UV-B辐射增强下施氮对大麦根际土壤酶活性的影响[J]. 土壤通报, 2013, 44(5): 1151-1157. LOU Yun-sheng, CHENG Huan-you, HAN Yan. Effect of enhanced ultraviolet-B radiation and nitrogen levels on enzymatic activities in barley rhizospheric soil[J]. Chinese Journal of Soil Science, 2013, 44(5): 1151-1157. |
| [39] |
张令瑄, 谢婷婷, 王瑾, 等. 大田条件下UV-B辐射增强对大豆根际土壤相关指标的影响[J]. 江苏农业学报, 2016, 32(1): 118-122. ZHANG Ling-xuan, XIE Ting-ting, WANG Jin, et al. Soybean rhizosphere soil parameters in response to enhanced UV-B radiation under field condition[J]. Jiangsu Journal of Agricultural Sciences, 2016, 32(1): 118-122. |
| [40] |
吴芳芳, 郑有飞, 吴荣军, 等. 太阳辐射减弱麦田根际土壤酶与有效微量元素的相关性分析[J]. 生态环境学报, 2012, 21(4): 595-600. WU Fang-fang, ZHENG You-fei, WU Rong-jun, et al. Correlative research on rhizosphere soil enzyme activities and available trace elements under reduced solar irradiance[J]. Ecology and Environmental Sciences, 2012, 21(4): 595-600. |
| [41] |
徐欣, 郑利远, 周珂, 等. 长期施肥对不同有机质水平黑土蛋白酶活性及氮素的影响[J]. 中国土壤与肥料, 2019(1): 44-48. XU Xin, ZHENG Li-yuan, ZHOU Ke, et al. Effects of long-term fertilization on protease activity and nitrogen in arable mollisols of different organic matter contents[J]. Soil and Fertilizer Sciences in China, 2019(1): 44-48. |
| [42] |
Tietjen T, Wetzel R G. Extracellular enzyme-clay mineral complexes: Enzyme adsorption, alteration of enzyme activity, and protection from photodegradation[J]. Aquatic Ecology, 2003, 37: 331-339. |
| [43] |
张威, 张明, 张旭东, 等. 土壤蛋白酶和芳香氨基酶的研究进展[J]. 土壤通报, 2008(6): 1468-1474. ZHANG Wei, ZHANG Ming, ZHANG Xu-dong, et al. A review on soil protease and arylamidase[J]. Chinese Journal of Soil Science, 2008(6): 1468-1474. |
| [44] |
陈意超, 李伏生, 李烙布. 不同灌溉方式和尿素猪粪比例对稻田氮素转化相关微生物活性的影响[J]. 华南农业大学学报, 2018, 39(1): 31-39. CHEN Yi-chao, LI Fu-sheng, LI Luo-bu. Effects of different irrigation methods and ratios of urea pig manure on microbial activity related to nitrogen transformation in paddy soil[J]. Journal of South China Agricultural University, 2018, 39(1): 31-39. |
| [45] |
封保根, 郭平, 李琳慧, 等. 纳米TiO2对土壤中硝态氮质量比及硝酸还原酶活性的影响[J]. 吉林大学学报(理学版), 2018, 56(6): 1570-1576. FENG Bao-gen, GUO Ping, LI Lin-hui, et al. Effect of nano-TiO2 on mass ratio of nitrate nitrogen and activity of nitrate reductase in soil[J]. Journal of Jilin University(Science Edition), 2018, 56(6): 1570-1576. |
| [46] |
方泽涛, 李伏生, 刘靖雯, 等. 不同灌溉模式和施氮处理下稻田N2O排放与反硝化酶活性的关系[J]. 应用与环境生物学报, 2017, 23(6): 1059-1066. FANG Ze-tao, LI Fu-sheng, LIU Jing-wen, et al. Relationship between N2O emission and denitrification enzyme activity in paddy soil under different irrigation modes and nitrogen treatments[J]. Chinese Journal of Applied and Environmental Biology, 2017, 23(6): 1059-1066. |
| [47] |
陈永勤, 冯勃, 徐卫红, 等. 小白菜硝酸盐含量与光照度及氮代谢关键酶的相关性[J]. 食品科学, 2016, 37(13): 183-188. CHEN Yong-qin, FENG Bo, XU Wei-hong, et al. Relationship between nitrate contents of Chinese cabbage(Brassica chinensis L.)and light intensity or key enzymes of nitrogen metabolism[J]. Food Science, 2016, 37(13): 183-188. |
| [48] |
Gao L M, Liu Y, Wang X F, et al. Lower levels of UV-B light trigger the adaptive responses by inducing plant antioxidant metabolism and flavonoid biosynthesis in Medicago sativa seedlings[J]. Functional Plant Biology, 2019, 46(10): 896-906. |
| [49] |
曹文超, 宋贺, 王娅静, 等. 农田土壤N2O排放的关键过程及影响因素[J]. 植物营养与肥料学报, 2019, 25(10): 1781-1798. CAO Wen-chao, SONG He, WANG Ya-jing, et al. Key production processes and influencing factors of nitrous oxide emissions from agricultural soils[J]. Journal of Plant Nutrition and Fertilizers, 2019, 25(10): 1781-1798. |
| [50] |
吴杨潇影, 姜振辉, 杨京平, 等. 玉米-水稻轮作和水稻连作土壤根际和非根际氮含量及酶活性[J]. 植物营养与肥料学报, 2019, 25(4): 535-543. WU Yang-xiao-ying, JIANG Zhen-hui, YANG Jing-ping, et al. Nitrogen content and enzyme activity in rhizosphere and non-rhizosphere soils of paddy field under maize-rice rotation and rice continuous mono-cropping[J]. Journal of Plant Nutrition and Fertilizers, 2019, 25(4): 535-543. |
| [51] |
马艳芹, 钱晨晨, 孙丹平, 等. 施氮水平对稻田土壤温室气体排放的影响[J]. 农业工程学报, 2016, 32(增刊2): 128-134. MA Yan-qin, QIAN Chen-chen, SUN Dan-ping, et al. Effect of nitrogen fertilizer application on greenhouse gas emissions from soil in paddy field[J]. Transactions of the CSAE, 2016, 32(Suppl 2): 128-134. |
| [52] |
韩佳乐, 郝珊, 刘振杰, 等. 黄土高原地区两种土地利用方式CO2和N2O排放特征[J]. 环境科学, 2019, 40(11): 5164-5172. HAN Jia-le, HAO Shan, LIU Zhen-jie, et al. Characteristics of CO2 and N2O emissions under two land use types in the Loess Plateau of China[J]. Environmental Science, 2019, 40(11): 5164-5172. |
| [53] |
祖艳群, 魏兰芳, 杨济龙, 等. 紫外辐射增加对40个割手密无性系土壤微生物种群数量动态和多样性的影响[J]. 农业环境科学学报, 2005, 24(1): 6-11. ZU Yan-qun, WEI Lan-fang, YANG Ji-long, et al. Effect of UV-B radiation on population dynamic and diversity of 40 wild sugarcane (Saccharum. spontaneum L.)clones rhizosphere microorganisms[J]. Journal of Agro-Environment Science, 2005, 24(1): 6-11. |
| [54] |
周贝贝, 王一明, 林先贵. 不同处理方式的粪肥对水稻生长和温室气体排放的影响[J]. 应用与环境生物学报, 2016, 22(3): 430-436. ZHOU Bei-bei, WANG Yi-ming, LIN Xian-gui. Effects of differently treated manures on rice growth and greenhouse gas emission[J]. Chin J Appl Environ Biol, 2016, 22(3): 430-436. |
 2020, Vol. 39
2020, Vol. 39