2. 农业农村部环境保护科研监测所, 农业农村部产地环境污染防控重点实验室, 天津市农业环境与农产品安全重点实验室, 天津 300191;
3. 华南农业大学资源环境学院, 广州 510642
2. Agro-Environmental Protection Institute, Ministry of Agriculture and Rural Affairs, Key Laboratory of Original Agro-Environmental Pollution Prevention and Control, Tianjin Key Laboratory of Agro-Environment and Agro-Product Safety, Tianjin 300191, China;
3. College of Natural Resources and Environment, South China Agricultural University, Guangzhou 510642, China
由土壤中植物病原菌引起的土传病害极大地威胁着全球的农产品质量与安全,在蔬菜种植土壤中尤为严重[1-2]。常见的土传病害包括病原真菌、病原细菌和线虫,易发生土传病害的蔬菜主要有茄子、番茄、芹菜、黄瓜和辣椒等[3]。对河南设施蔬菜大棚的调查发现,土传病害严重程度随着蔬菜连作年限的延长而增大,病害种类主要包括根结线虫病、根腐病、茎基腐病和青枯病,发病率分别达85%、54%、26%和18%[4]。因此,采取有效措施降低植物土传病害至关重要。
生物防治和化学土壤熏蒸消毒是当前防治植物土传病害的两大关键技术[3, 5],其中生物防治技术主要由有益微生物介导,通过浸种、蘸根、混土等途径接种[6]。目前已经成功研发多种生物防治剂如产紫篮状菌(Talaromyces sp.)[7]、木霉菌(Trichoderma sp.)[8]和芽孢杆菌(Bacillus sp.)[9],并实现了对土传病害的预防。然而,虽然生物防治具有环境友好性,但该技术对土传病害相对较弱的防治效果导致它目前很难完全替代土壤熏蒸消毒技术。
土壤熏蒸剂是指施入土壤后能够产生具有杀灭农业病、虫、草害能力的气体,从而在人为密闭环境中防治土传病害的一类农药[10]。继溴甲烷被禁止使用后,威百亩、氯化苦、二甲基二硫和1,3-二氯丙烯等物质成为替代溴甲烷的常见土壤熏蒸剂,且在防治土传病害方面取得了显著的成效[11-13]。例如,采用滴灌法将氯化苦(40 g·m-2)施入种植辣椒的土壤后,对辣椒黄萎病的控制效率达80%~96%,同时辣椒根腐病也得到有效控制[14]。采用威百亩+二甲基二硫组合熏蒸方式能够极大地抑制黄瓜病原菌(如Meloidogyne sp.、Fusarium sp. 和Phytophthora sp.)的丰度,黄瓜产量和经济收入较对照处理分别增加了77%~123% 和100%~130%[15]。
土壤熏蒸剂也可能在一定程度上引起环境的污染。一方面,由于大部分替代溴甲烷的熏蒸剂依旧具有一定的挥发性,这会对大气环境造成潜在的威胁[16]。然而,通过薄膜覆盖、水封、滴灌、添加生物炭等措施可很大程度地降低其向大气环境的释放[17-19]。例如,铁改性生物炭可以活化过氧化尿素产生·OH自由基,从而有效促进吸附在生物炭上的1,3-二氯丙烯的降解(其残余的顺式异构体从33% 下降至11%)[19]。这将有助于降低土壤熏蒸技术对大气环境的危害,从而增强该技术的环境友好性。
另一方面,熏蒸剂也可能会对土壤环境造成一定影响,如引起土壤酸化、抑制酶活性等[20-21]。同时,土壤微生物群落是土壤生态系统中的重要组成部分,它们除作为植物病原菌的主要源头外,其中的有益菌还能够促进土壤养分循环[22],参与土壤污染修复[23]。由于现有的大部分熏蒸剂(如威百亩和氯化苦)均具有广谱杀菌性,导致土壤熏蒸过程也可能会抑制部分有益菌的活性,如硝化细菌和反硝化细菌[24-25]。也有研究指出,虽然氯化苦熏蒸抑制了氨氧化古菌活性,但氨氧化细菌活性得到了增强[26]。此外,1,3-二氯丙烯熏蒸改变了固氮细菌、硝化细菌和反硝化细菌在土壤中的组成,虽然在短期内该熏蒸剂降低了土壤中固氮、硝化和反硝化功能基因的丰度,但氮循环功能基因丰度在试验后期又不同程度地恢复到未熏蒸水平[27]。近期研究表明,氯化苦熏蒸可提高参与磷循环微生物的丰度,有利于土壤中磷的释放[28]。
土壤真菌是植物病原菌的重要来源,如Fusarium sp.[29]、Pythiums sp. [30]和Gibberella sp.[31]。先前研究表明,熏蒸剂能够明显抑制土壤真菌群落的活性,且真菌群落丰度随时间的推移很难再恢复至原来的水平[32-34]。然而,由土壤熏蒸造成真菌群落这种“难以恢复式”的演变趋势是否有利于土壤生态系统的发展有待深入研究。同时,微生物在土壤环境中并非独立生存,相反它们之间复杂的种间关系造就了一个密集的代谢网络[35]。目前为止,土壤熏蒸后真菌群落中病原真菌与其他功能菌间种间关系的变化规律需进一步加以阐明。
本研究采集黄瓜大棚连作3年以上的土壤,选取威百亩作为典型的土壤熏蒸剂,并设置不同的熏蒸浓度和采样时间梯度,采用高通量测序技术研究威百亩熏蒸土壤后不同功能菌群的演变特征,探明优势菌、有益菌与病原真菌之间的内在联系,以期为土壤中土传病害的防控提供科学依据。
1 材料与方法 1.1 供试材料供试土壤为褐土,采自山西省太原市小店区黄瓜大棚连作3年以上的耕层土壤(0~20 cm,过2 mm筛),经调查该土壤无威百亩熏蒸史。土壤pH为6.98,有机质和全氮含量分别为24.85 g·kg-1和1.27 g· kg-1,碱解氮、有效磷和速效钾含量分别为50.08、43.96 mg·kg-1和223 mg·kg-1。
威百亩的化学名称为N-甲基二硫代氨基甲酸钠,分子式为C2H4NNaS2,易溶于水(772 g·L-1,20 ℃),难溶于大多数有机溶剂,在酸和重金属盐存在的条件下易分解[10],其分子结构见图 1。它通过释放出异硫氰酸甲酯(C2H3NS)等物质阻断生物体内细胞分裂和DNA、RNA以及蛋白质的合成过程,从而达到防治病虫害的目的[11]。异硫氰酸甲酯的半衰期在9.93~10.51 h[34],这将在很大程度上降低由威百亩熏蒸对土壤环境造成的持续威胁。本研究使用的威百亩为42%的水剂,购自山东金畅鑫化工科技有限公司。
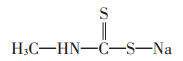
|
图 1 威百亩的分子结构 Figure 1 Molecular structure of metam sodium |
采用微宇宙模式进行土壤熏蒸试验,具体方法如下[34]:将300 g供试土壤置于棕色广口瓶中,分别设定高浓度威百亩熏蒸处理(HM,100 mg·kg-1)、低浓度威百亩熏蒸处理(LM,50 mg·kg-1)和未熏蒸对照处理(NM),每个处理9个平行。添加威百亩后,将广口瓶进行密封处理,并在恒温(25 ℃)培养箱中避光培养7 d。7 d后打开广口瓶盖,随后再用透气膜将瓶口封好,并继续在避光恒温条件下培养,培养周期为40 d(不包括最开始7 d的密闭熏蒸过程),在整个试验周期,所有处理土壤含水量均保持最大持水量的60%。同时,分别在试验的第3、15 d和40 d进行破坏性取样(每次随机抽取3个平行),取出的样品经液氮处理后保存至-80 ℃冰箱中待用,以测定土壤熏蒸后真菌群落的变化规律。
1.3 测定方法 1.3.1 土壤DNA的提取使用Power Soil试剂盒提取土壤中的DNA(每个样品约需0.5 g土壤),具体方法按照试剂盒提供的说明书进行。DNA的纯度和浓度通过琼脂糖凝胶电泳检测,将适量样品置于离心管中,并用无菌水将待测样品稀释至1 ng·μL-1。
1.3.2 PCR扩增将稀释后的基因组DNA进行PCR扩增,PCR反应程序如下:98 ℃预变性1 min;30个循环包括98 ℃,10 s;50 ℃,30 s;72 ℃,30 s;在72 ℃条件下最终延伸5 min。真菌的扩增引物为ITS1F(5′-CTTGGTCATTTAGAGGAAGTAA -3′)和ITS2R(5′ - GCTGCGTTCTTCATCGATGC-3′)[36]。
1.3.3 PCR产物的鉴定、纯化与定量基于PCR产物浓度的结果进行混样处理,随后用2% 的琼脂糖胶电泳对PCR的产物进行检测。使用AxyPrepDNA对PCR产物进行纯化,主要步骤如下:将包含目的DNA的琼脂糖凝胶在紫外灯下切下并计质量,分别先后混入Buffer DE-A和Buffer DE-B,混合液经过离心、洗脱等步骤后,再用QuantusTM Fluorometer对PCR产物进行检测定量。
1.3.4 文库构建与上机测序构建文库所用的试剂盒购自Thermofisher公司,待文库构建完成后需检测是否合格(Qubit定量),文库检测通过后进行样品上机测序步骤(上海美吉生物医药科技有限公司),待测序完成后进行数据处理(主要包括Reads的拼接、Tags数据的过滤和质控、OTUs的聚类及后续的信息挖掘)。
1.4 数据统计分析使用软件Excel 2007进行数据分析,采用软件SPSS Statistics 2.2进行组间差异分析(单因素方差分析和t检验),结合美吉生物云平台和软件Origin 2019制图(Network图除外)。运用软件Cytoscape 3.7.1进行Network关联分析,用于揭示土壤真菌群落种间关系的变化规律(先借助软件SPSS Statistics 2.2对属水平真菌进行Spearman相关性分析,筛选出显著相关(P < 0.05)的两两关系再进行制图,以提高Network的可读性)。
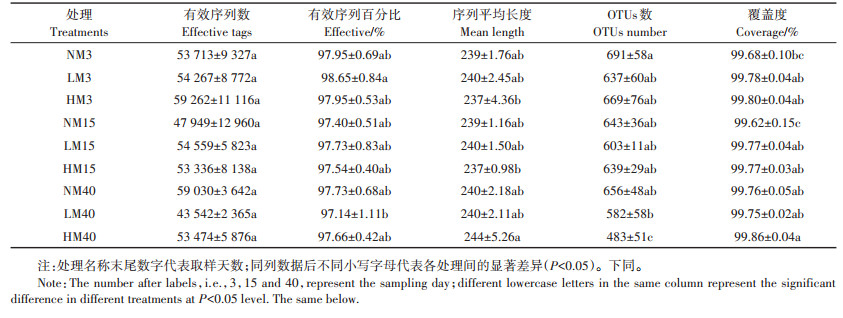
2 结果与分析 2.1 威百亩对土壤真菌多样性的影响通过对27个土壤样品真菌群落进行测序,总共得到1 437 395条有效序列,各样品平均53 237条reads,平均长度为237~244 bp(表 1)。测序序列在97% 的相似度水平下被归类后,比对出481~691个OTUs。覆盖度均高于99%,说明测序深度能够很好地反映真菌群落的状况。
|
|
表 1 真菌群落的测序信息统计 Table 1 Sequencing data of fungal community |
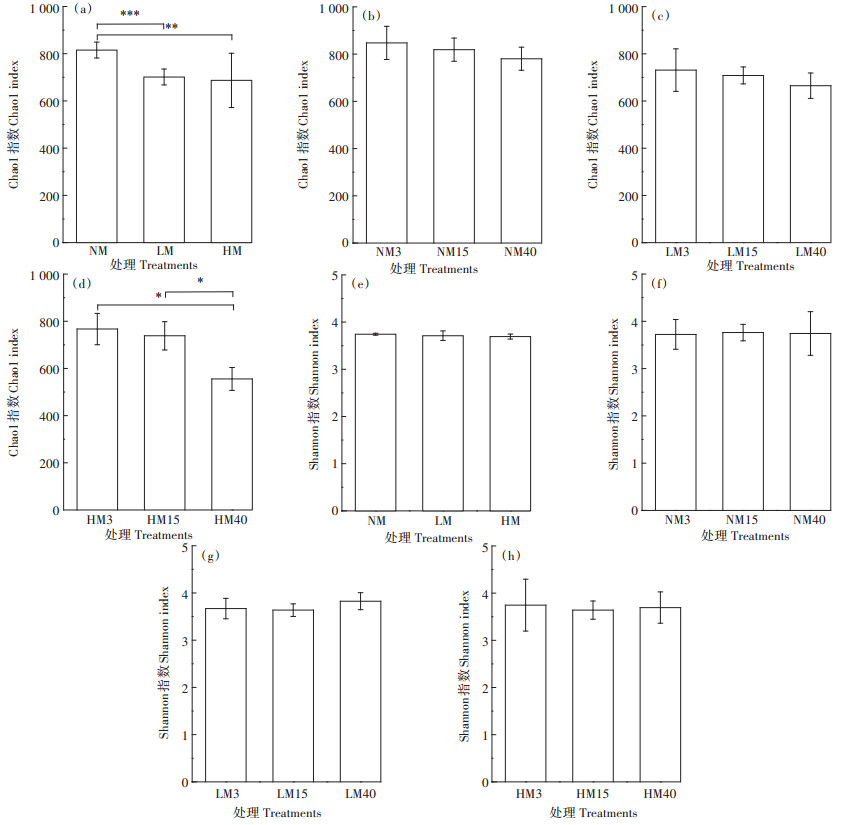
从总体来看,不同处理间Shannon指数几乎没有差别(图 2e),而经过威百亩熏蒸后Chao1指数较对照处理显著降低了14%~16%(P < 0.01,图 2a),说明威百亩熏蒸可以抑制土壤中真菌群落的丰度。同时,在NM和LM处理中,Chao1和Shannon指数均随时间推移没有显著的变化(图 2b~图 2c和图 2f~图 2g)。然而,Chao1指数在HM处理中随时间而逐渐降低,且在第40 d较第3 d显著下降了28%(P < 0.05,图 2d),说明高剂量的威百亩熏蒸对土壤真菌群落的影响更为明显。
|
*、**和***分别代表两组间的显著差异达0.01 < P≤0.05、0.001 < P≤0.01和P≤0.001水平。下同 The symbols, *, ** and ***, represent significant differences between two groups at 0.01 < P≤0.05, 0.001 < P≤0.01 and P≤0.001 level, respectively. The same below 图 2 Chao1指数和Shannon指数在不同处理、不同时间下的变化 Figure 2 Change of Chao1 and Shannon indexes in different treatments and with different time |
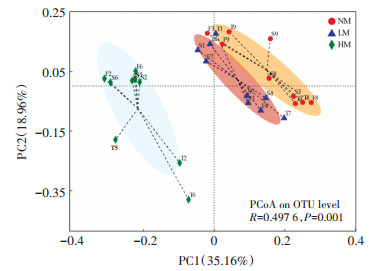
在OTUs分类水平下,采用Bray-Curtis距离算法对不同处理的真菌群落进行了主坐标分析(PCoA),两排序轴的总解释度达54.12%(图 3)。聚类结果显示,不同处理间的真菌群落分别各自聚集在了一起,其中HM处理的聚类效果尤为明显。进一步通过ANOSIM检验发现,组间差异达显著水平(P < 0.01),说明威百亩熏蒸显著改变了土壤真菌群落的结构。
|
图 3 真菌群落的PCoA分析 Figure 3 PCoA analysis of fungal community |
由图 4可知,供试土壤中丰度排名前5的门水平真菌依次分别为Ascomycota(84%~89%)、Mortierellomycota(2.9%~11%)、unclassified_k_Fungi(1.8%~4.4%)、Basidiomycota(1.4%~3%)和Chytridiomycota(0.7%~2.4%)。总体来看,与未熏蒸处理相比,威百亩熏蒸后Ascomycota、unclassified_k_Fungi和Chytridiomycota丰度呈增加趋势,而Mortierellomycota在LM和HM处理中分别降低了45% 和75%(图 4a~图 4c)。随着时间的推移,Ascomycota丰度在NM处理中逐渐降低,而在HM处理中逐渐升高,unclassified_k_Fungi丰度展现出与Ascomycota相同的趋势(图 4d)。对于Mortierellomycota,它的丰度在NM和LM处理中随着时间的推移几乎没有变化,而在HM处理中随时间推移呈现出明显的降低趋势,其降幅达46%~89%。

|
图 4 相对丰度排名前5的真菌(门水平)在不同处理中的变化及随时间的演变规律 Figure 4 Abundance of fungal community(top 5)at the phylum level in different treatments, and their evolution with time |
属水平相对丰度排名前35的真菌群落的聚类热图见图 5,由图可知,排名前5的属水平真菌分别依次为unclassified_f_Microascaceae、unclassified_o_Sordariales、Mortierella、unclassified_p_Ascomycota和Cephaliophora。总体来看,威百亩熏蒸后unclassified_f_Microascaceae和unclassified_p_Ascomycota丰度分别增加了20%~62% 和17%~127%,且其丰度增幅随威百亩熏蒸浓度的增加而升高(图 5a)。同时,Talaromyces、Arthrographis、Acaulium、unclassified_k_Fungi、Paracremonium和unclassified_o_Coniochaetales丰度展现出与unclassified_f_Microascaceae相同的趋势。例如,与NM处理相比,Talaromyces丰度在LM和HM处理中分别升高了41% 和186%。此外,虽然低浓度威百亩熏蒸对Microascus和Leucothecium丰度的影响较小,但在高浓度威百亩熏蒸处理中其丰度较对照分别增加了92%和85%。

|
图 5 相对丰度排名前35的真菌(属水平)在不同处理中的变化及其随时间的演变规律 Figure 5 The abundance of fungal community(top 35)at the genus level and their evolution with time in different treatments |
相反,unclassified_o_Sordariales、Mortierella和Cephaliophora丰度在威百亩熏蒸后分别降低了16%~61%、44%~74% 和7%~99%,其降低幅度随威百亩熏蒸浓度的增加而增大(图 5a)。同时,Gibberella、Chaetomium、Cheilymenia、Fusarium、Subramaniula和Phoma丰度展示出与unclassified_o_Sordariales相同的趋势。例如,Gibberella丰度在LM和HM处理中较NM处理分别降低了26%和95%。
随着时间的推移,unclassified_p_Ascomycota、Talaromyces、Arthrographis、unclassified_k__Fungi、Paracremonium和unclassified_o_Coniochaetales丰度在未熏蒸处理中变幅较小,而在高浓度威百亩熏蒸处理中呈明显的增加趋势(图 5b)。例如,Talaromyces和unclassified_p_Ascomycota丰度在第40 d较第3 d分别提升了100% 和146%。然而,Mortierella丰度在NM处理中随时间逐渐升高,而在LM和HM处理中随时间逐渐降低,尤其在HM处理中,其丰度在第40 d较第3 d降低了90%。值得关注的是,在LM处理中,Cephaliophora、Gibberella和Fusarium丰度虽然在实验初期较未熏蒸对照处理有不同程度的降低,但随着时间的推移它们的丰度又呈现出逐渐升高的趋势,且在第40 d较第3 d分别提高了82%、64% 和89%。相比之下,在HM处理中,Cephaliophora、Gibberella和Fusarium丰度随时间推移展现出明显的降低趋势。
2.3 物种差异分析与功能预测采用单因素方差分析对属水平真菌群落进行多组间差异显著性检验,结果表明,除Phoma、unclassified_o_Sordariales、Leucothecium和unclassified_p_Ascomycota外,2.2.2节中所提到的相对丰度在不同组间存在明显变幅的属水平真菌,如Mortierella、Cephaliophora、Talaromyces、Fusarium和Microascus等,其变幅差异均达显著水平(图 6a)。为了进一步研究威百亩熏蒸后真菌群落结构的变化特征,选取高浓度威百亩熏蒸处理(HM)和未熏蒸对照处理(NM)进行了LEfSe物种差异判别分析和线性判别分析(LDA,阈值为3,多比较策略),研究结果见图 6b。由图可知,在NM处理中具有代表性的属水平真菌(排名前35)按其影响力从大到小依次为Mortierella、Cephaliophora、Gibberella、Chaetomium、Cheilymenia、Cutaneotrichosporon、unclassified_c_Sordariomycetes、Metarhizium和Fusarium。而HM处理中的代表性物种依次为unclassified_f_Microascaceae、unclassified_k_Fungi、unclassified_p_Ascomycota、Microascus、Talaromyces、unclassified_o_Coniochaetales、Acaulium、Paracremonium、Leucothecium、Arthrographis和unclassified_p_Chytridiomycota。这再次说明威百亩熏蒸改变了土壤真菌的群落结构。
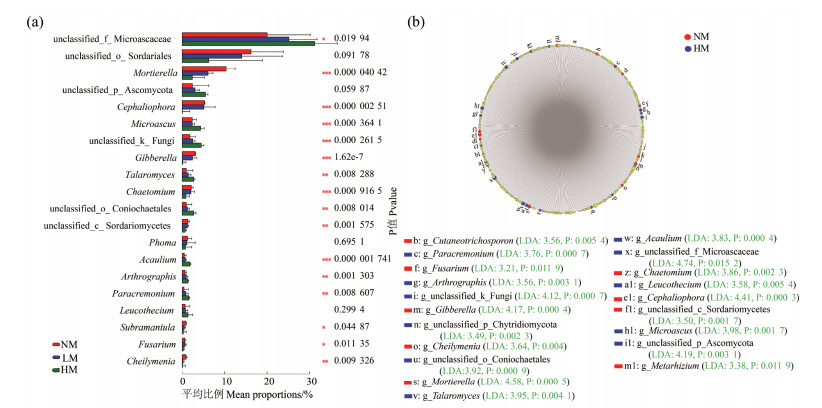
|
图 6 相对丰度排名前35真菌(属水平)的组间差异检验及威百亩熏蒸后代表物种的变化 Figure 6 Difference test of fungal community at the genus level(top 35)among groups and changes of key species after fumigation with metam sodium |
借助FUNGuild对真菌群落中的潜在植物病原菌进行了预测[37],主要包括Gibberella intricans、Gibberella avenacea、unclassified_g_Clonostachys、unclassified_g_Volutella、Gibellulopsis nigrescens、unclassified_g_Curvularia、Neonectria candida、Clonostachys epichloe和Lectera sp,从总体上看,威百亩熏蒸土壤后潜在植物病原菌活性受到了显著抑制(其丰度较对照处理降低了30%~50%,P < 0.05),且高浓度威百亩熏蒸对潜在病原菌的抑制效果更明显(图 7)。

|
图 7 潜在植物病原菌在不同处理中的变化 Figure 7 Changes of potential plant pathogens in different treatments |
为了进一步探明威百亩熏蒸对土壤真菌群落的影响,选取HM和NM处理中各自典型的属水平真菌(见2.3节,共21个)进行Network关联分析,以揭示威百亩熏蒸后土壤真菌种间关系的变化规律(图 8)。由其网络拓扑参数可知,在NM处理中,不仅总边数较HM处理高出27%,而且最短路径在NM处理中较HM也高出54%,说明威百亩熏蒸后在一定程度上降低了物种之间的关联度。有趣的是,在NM处理中节点(属水平真菌)两两之间均为正相关关系(图 8a),而在威百亩熏蒸后出现了负相关,且占比达总边数39%(图 8b),说明威百亩熏蒸改变了土壤真菌群落的种间关系。经过高浓度威百亩熏蒸后,土壤中unclassified_f_Microascaceae与Cheilymenia、Fusarium与unclassified_k_Fungi和Leucothecium、Gibberella与Leucothecium之间的种间关系均从正相关转变为负相关。此外,在HM处理中,Cephaliophora、Cheilymenia、Gibberella和Fusarium均分别与多个属水平真菌展现出负相关关系,如Fusarium与unclassified_p_Ascomycota、unclassified_k_Fungi和Leucothecium之间的负相关。
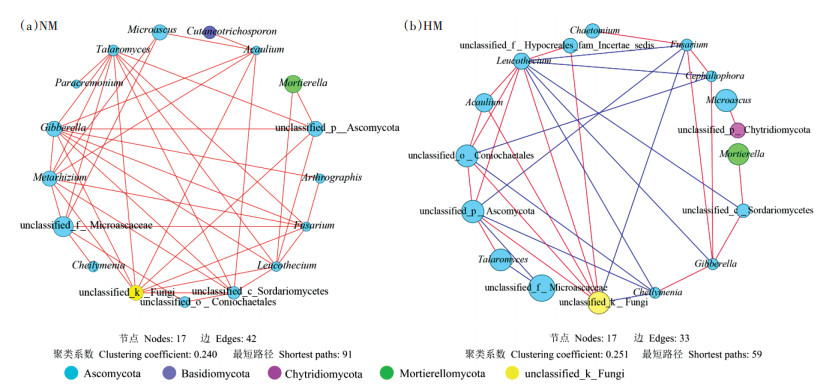
|
红线代表两两之间的显著正相关关系,蓝线代表显著负相关关系(P < 0.05),节点的大小与微生物的丰度呈正比 Red edges represent significant positive relationships and blue edges represent significant negative relationships(P < 0.05), size of each node is proportional to the abundance of the corresponding fungi 图 8 属水平真菌在未熏蒸处理和高浓度威百亩熏蒸处理中的Network关联分析 Figure 8 Network correlation analysis of fungal community at the genus level in the non-fumigation treatment and high-concentrated metam sodium fumigation treatment |
在属水平排名前35的真菌中,除Gibberella sp. 外,Fusarium sp.也是常见的病原真菌,它不仅会导致小麦、玉米等粮食作物根腐、茎腐、萎蔫[38-39],而且还会引起黄瓜枯萎病[40-41],已经造成全球性的危害。在本研究中,Gibberella和Fusarium丰度均随威百亩熏蒸浓度的增加而显著降低。
先前研究发现,土壤细菌多样性在短期内会受到威百亩熏蒸的抑制,且这种抑制效果会随威百亩熏蒸剂量的增加更为明显,但其多样性最终会随着时间的推移逐渐恢复[42]。当威百亩熏蒸剂量低于12 mg·kg-1时,对真菌数量的抑制效果随时间的推移逐渐减弱,但当威百亩熏蒸浓度高于90 mg·kg-1后,对真菌数量的抑制率在整个实验周期始终高达100%[32]。在本研究中,威百亩熏蒸后土壤真菌的群落结构发生了明显的变化,且这种差异随着威百亩熏蒸浓度的增加而增大。对于Gibberella和Fusarium,在低剂量(50 mg · kg-1)威百亩熏蒸处理中,它们经历了抑制-恢复等系列过程,但在高剂量(100 mg·kg-1)处理中,以上2种潜在病原真菌始终被抑制。因此,选择高浓度(100 mg·kg-1)威百亩熏蒸能够相对更彻底地清除土壤中的病原真菌。此外,Chaetomium sp. 是一种抑制植物病害的生物防治菌,它能够产生多种生物活性代谢物质从而降低黄瓜菌核病、马铃薯晚疫病和芸薹属蔬菜的发病率[43-44]。在本研究中,Chaetomium丰度在威百亩熏蒸后显著降低,这与前人的研究结果一致,这可能是土壤微生物的多样性和复杂性掩盖了它在疾病控制中的作用[45]。
秸秆还田是当今社会农业生产中常见的农作措施,它具有改善环境和培肥地力的双功效,然而纤维素等难溶物质的分解成为限制秸秆降解转化的关键步骤。近期研究表明,Talaromyces sp. 能够通过分泌内切葡聚糖酶、纤维二糖水解酶、β-葡萄糖苷酶和木聚糖酶从而促进秸秆中纤维素的降解转化[46]。在本研究中,Talaromyces的丰度随威百亩熏蒸浓度的增加呈显著的增长趋势,在实验第40 d,其丰度排名从第19位(NM处理)上升至第7位(HM处理),这将会在一定程度上有利于增强土壤肥力。此外,Talaromyces sp. 也可以作为生物防治苦瓜枯萎病的有益菌[7]。檀兴燕[47]研究发现,加入高浓度灭菌剂后,Arthrographis sp. 在种植番茄土壤中大量繁殖,这与本研究的结果一致。最新的研究表明,Arthrographis sp.具有分泌脂肪酶的能力[48],一方面该酶可以促进糖类生成从而为作物种子萌发提供能量,另一方面该酶能够作为降解功能酶促进土壤中有机污染物的降解[49]。总之,威百亩熏蒸不仅可以有效抑制土壤中的病原真菌,而且还有利于土壤中Talaromyces sp.和Arthrographis sp. 等有益菌的生长。
通过Network关联分析发现,虽然威百亩熏蒸后真菌群落之间的关联度有所降低,但是出现了潜在病原菌(Gibberella、Cephaliophora和Fusarium)与优势菌(unclassified_o_Coniochaetales、unclassified_k_Fungi和unclassified_p_Ascomycota,在试验第40 d的HM处理中丰度排名前5)和其他功能菌(Leucothecium,排名第9)之间的负相关关系,这意味着它们之间可能存在着竞争、捕食和偏害等种间关系。换而言之,很可能潜在病原菌会受到Leucothecium等上述提到的4种属水平真菌的拮抗作用,从而降低它们在土壤生态系统中的生态位宽度,最终抑制潜在病原菌的新陈代谢,有利于提高作物的产量和品质。此外,曾有研究指出Leucothecium sp.可以抑制蚊虫幼虫的生长[50],而该菌在生物防治植物病原菌过程中起到何种作用还需进行后续的研究。
4 结论(1)潜在病原真菌受到高浓度(100 mg·kg-1)威百亩熏蒸的显著抑制,其丰度随时间推移逐渐降低。
(2)虽然威百亩熏蒸后Chaetomium sp. 没有展现出其应有的生物防治作用,但是某些与土壤肥力相关的有益菌和降解功能菌(Talaromyces sp.和Arthrographis sp.)活性得到了强化。
(3)威百亩熏蒸改变了真菌群落的种间关系,优势菌对病原真菌的拮抗作用降低了病原真菌在土壤生态系统中的生态位宽度。
| [1] |
蔡祖聪, 黄新琦. 土壤学不应忽视对作物土传病原微生物的研究[J]. 土壤学报, 2016, 53(2): 305-310. CAI Zu-cong, HUANG Xin-qi. Soil-borne pathogens should not be ignored by soil science[J]. Acta Pedologica Sinica, 2016, 53(2): 305-310. |
| [2] |
De Corato U. Disease-suppressive compost enhances natural soil sup pressiveness against soil-borne plant pathogens: A critical review[J]. Rhizosphere, 2020, 13: 100192. DOI:10.1016/j.rhisph.2020.100192 |
| [3] |
曹坳程, 刘晓漫, 郭美霞, 等. 作物土传病害的危害及防治技术[J]. 植物保护, 2017, 43(2): 6-16. CAO Ao-cheng, LIU Xiao-man, GUO Mei-xia, et al. Incidences of soil-borne diseases and control mea sures[J]. Plant Protection, 2017, 43(2): 6-16. DOI:10.3969/j.issn.0529-1542.2017.02.002 |
| [4] |
王广印, 郭卫丽, 陈碧华, 等. 河南省设施蔬菜连作障碍现状调查与分析[J]. 中国农学通报, 2016, 32(25): 27-33. WANG Guang-yin, GUO Wei-li, CHEN Bi-hua, et al. Continuous cropping obstacles of fa cilities vegetables in Henan: Investigation and analysis[J]. Chinese Agricultural Science Bulletin, 2016, 32(25): 27-33. DOI:10.11924/j.issn.1000-6850.casb16060130 |
| [5] |
孙广正, 姚拓, 刘婷, 等. 植物根际促生菌对3种土传真菌病害病原的抑制作用[J]. 微生物学通报, 2014, 41(11): 2293-2300. SUN Guang-zheng, YAO Tuo, LIU Ting, et al. Antagonism of plant growth promoting rhizobacteria on three soil-borne fungous pathogen[J]. Microbiology China, 2014, 41(11): 2293-2300. |
| [6] |
Mancini V, Romanazzi G. Seed treatments to control seedborne fungal pathogens of vegetable crops[J]. Pest Management Science, 2014, 70(6): 860-868. DOI:10.1002/ps.3693 |
| [7] |
田叶韩, 彭海莹, 王德浩, 等. 产紫篮状菌的生防潜力及其对土壤微生物群落的调控[J]. 应用生态学报, 2020, 31(10): 3255-3266. TIAN Ye-han, PENG Hai-ying, WANG De-hao, et al. Biocontrol po tential of Talaromyces purpurogenus and its regulation on soil microbial community[J]. Chinese Journal of Applied Ecology, 2020, 31(10): 3255-3266. |
| [8] |
朱萍萍, 凌健, 席亚东, 等. 蔬菜土传病害生防木霉菌株资源的筛选及其防治效果评价[J]. 中国蔬菜, 2015, 8: 28-33. ZHU Ping-ping, LING Jian, XI Ya-dong, et al. Screening and bio-control effect assess ment of Trichoderma strains against 3 soilborn vegetable pathogens[J]. China Vegetables, 2015, 8: 28-33. |
| [9] |
Jiang C H, Yao X F, Mi D D, et al. Comparative transcriptome analysis reveals the biocontrol mechanism of Bacillus velezensis F21 against Fu sarium wilt on watermelon[J]. Frontiers in Microbiology, 2019, 10: 652. DOI:10.3389/fmicb.2019.00652 |
| [10] |
王秋霞, 颜冬冬, 王献礼, 等. 土壤熏蒸剂研究进展[J]. 植物保护学报, 2017, 44(4): 529-543. WANG Qiu-xia, YAN Dong-dong, WANG Xian-li, et al. Research advances in soil fumigants[J]. Journal of Plant Protection, 2017, 44(4): 529-543. |
| [11] |
李青杰, 方文生, 颜冬冬, 等. 熏蒸剂对土壤微生物的影响研究进展[J]. 农药学学报, 2019, 21(5-6): 780-786. LI Qing-jie, FANG Wen-sheng, YAN Dong-dong, et al. Research progress on the effect of fumigants on soil microorganisms[J]. Chinese Journal of Pesticide Science, 2019, 21(5/6): 780-786. |
| [12] |
Davison E M, Kazemi S, McDonald S, et al. Use of metham sodium to eliminate Phytophthora spp. from roading gravel[J]. Australasian Plant Pathology, 2019, 48(6): 563-572. DOI:10.1007/s13313-019-00660-0 |
| [13] |
Fang W S, Wang X L, Huang B, et al. Comparative analysis of the ef fects of five soil fumigants on the abundance of denitrifying microbes and changes in bacterial community composition[J]. Ecotoxicology and Environmental Safety, 2020, 187: 109850. DOI:10.1016/j.ecoenv.2019.109850 |
| [14] |
Ślusarski C, Spotti C A. Efficacy of chloropicrin application by drip ir rigation in controlling the soil-borne diseases of greenhouse pepper on commercial farms in Poland[J]. Crop Protection, 2016, 89: 216-222. DOI:10.1016/j.cropro.2016.07.024 |
| [15] |
Mao L, Jiang H Y, Zhang L, et al. Assessment of the potential of a re duced dose of dimethyl disulfide plus metham sodium on soilborne pests and cucumber growth[J]. Scientific Reports, 2019, 9: 19806. DOI:10.1038/s41598-019-56450-7 |
| [16] |
严丽丽. 量子点敏化TiO2复合薄膜的制备及其对土壤熏蒸剂大气散发的控制[D]. 上海: 上海交通大学, 2017: 1-4. YAN Li-li. The preparation of TiO2 composite film sensitized with quantum dots and its ability to reduce emission of soil fumigant into atmosphere[D]. Shanghai: Shanghai Jiaotong University, 2017: 1-4. |
| [17] |
Xuan R, Yates S R, Ashworth D J, et al. Mitigating 1, 3-dichloropro pene, chloropicrin, and methyl iodide emissions from fumigated soil with reactive film[J]. Environmental Science & Technology, 2012, 46(11): 6143. |
| [18] |
Ashworth D J, Luo L, Xuan R, et al. Irrigation, organic matter addi tion, and tarping as methods of reducing emissions of methyl iodide from agricultural soil[J]. Environmental Science & Technology, 2011, 45(4): 1384. |
| [19] |
Qin J L, Ashworth D J, Yates S R, et al. Coupled use of Fe-impregnat ed biochar and urea-hydrogen peroxide to simultaneously reduce soil–air emissions of fumigant and improve crop growth[J]. Journal of Hazardous Materials, 2020, 396: 122762. DOI:10.1016/j.jhazmat.2020.122762 |
| [20] |
Cheng H Y, Zhang D Q, Huang B, et al. Organic fertilizer improves soil fertility and restores the bacterial community after 1, 3-dichloro propene fumigation[J]. Science of the Total Environment, 2020, 738: 140345. DOI:10.1016/j.scitotenv.2020.140345 |
| [21] |
Klose S, Acosta-Martínez V, Ajwa H A. Microbial community compo sition and enzyme activities in a sandy loam soil after fumigation with methyl bromide or alternative biocides[J]. Soil Biology and Biochemistry, 2006, 38(6): 1243-1254. DOI:10.1016/j.soilbio.2005.09.025 |
| [22] |
任凤玲, 张旭博, 孙楠, 等. 施用有机肥对中国农田土壤微生物量影响的整合分析[J]. 中国农业科学, 2018, 51(1): 119-128. REN Feng-ling, ZHANG Xu-bo, SUN Nan, et al. A meta-analysis of ma nure application impact on soil microbial biomass across China ' s croplands[J]. Scientia Agricultura Sinica, 2018, 51(1): 119-128. |
| [23] |
Zhao X D, Li X J, Li Y, et al. Shifting interactions among bacteria, fungi and archaea enhance removal of antibiotics and antibiotic resis tance genes in the soil bioelectrochemical remediation[J]. Biotechnology for Biofuels, 2019, 12: 160. DOI:10.1186/s13068-019-1500-1 |
| [24] |
燕平梅, 魏爱丽, 赵文婧, 等. 氯化苦熏蒸对土壤反硝化作用及nirS型反硝化细菌群落结构的影响[J]. 植物保护, 2019, 45(6): 208-213. YAN Ping-mei, WEI Ai-li, ZHAO Wen-jing, et al. Ef fects of chloropicrin fumigation on soil denitrification and the commu nity structure of nirS-denitrifying bacteria[J]. Plant Protection, 2019, 45(6): 208-213. |
| [25] |
燕平梅, 李娜, 赵文婧, 等. 威百亩熏蒸对土壤硝化作用及amoA型硝化细菌群落结构的影响[J]. 植物保护学报, 2019, 46(6): 1357-1363. YAN Ping-mei, LI Na, ZHAO Wen-jing, et al. Effects of metham sodium fumigation on soil nitrification and the community structure of amoA nitrification bacteria[J]. Journal of Plant Protection, 2019, 46(6): 1357-1363. |
| [26] |
Li J, Huang B, Wang Q X, et al. Effect of fumigation with chloropicrin on soil bacterial communities and genes encoding key enzymes in volved in nitrogen cycling[J]. Environmental Pollution, 2017, 227: 534-542. DOI:10.1016/j.envpol.2017.03.076 |
| [27] |
Fang W S, Yan D D, Wang Q X, et al. Changes in the abundance and community composition of different nitrogen cycling groups in response to fumigation with 1, 3-dichloropropene[J]. Science of the Total Environment, 2018, 650(Pt 1): 44-55. |
| [28] |
Huang B, Yan D D, Ouyang C B, et al. Chloropicrin fumigation alters the soil phosphorus and the composition of the encoding alkaline phosphatase phoD gene microbial community[J]. Science of the Total Environment, 2020, 711: 135080. DOI:10.1016/j.scitotenv.2019.135080 |
| [29] |
Moura R D, Castro Lamd, Culik M P, et al. Culture medium for im proved production of conidia for identification and systematic studies of Fusarium pathogens[J]. Journal of Microbiological Methods, 2020, 173: 105915. DOI:10.1016/j.mimet.2020.105915 |
| [30] |
Hendrix F F, Campbell W A. Pythiums as plant pathogens[J]. Annual Review of Phytopathology, 1973, 11(1): 77-98. DOI:10.1146/annurev.py.11.090173.000453 |
| [31] |
Dalla Lana F, Paul P A, Minyo R, et al. Stability of hybrid maize reac tion to gibberella ear rot and deoxynivalenol contamination of grain[J]. Phytopathology, 2020, 110(12): 1908-1922. DOI:10.1094/PHYTO-05-20-0194-R |
| [32] |
卜东欣, 张超, 张鑫, 等. 熏蒸剂威百亩对土壤微生物数量和酶活性的影响[J]. 中国农学通报, 2014, 30(15): 227-233. BU Dong-xin, ZHANG Chao, ZHANG Xin, et al. Effects of fumigant metham-sodium on soil microbial population and enzyme activities[J]. Chinese Agricultural Science Bulletin, 2014, 30(15): 227-233. DOI:10.11924/j.issn.1000-6850.2013-2813 |
| [33] |
王小星, 樊文华. 熏蒸剂对土传病害防治的研究进展[J]. 山西农业大学学报(自然科学版), 2017, 37(1): 72-76. WANG Xiao-xing, FAN Wen-hua. The research progress of fumigant' s effects to soil-borne disease prevention and treatment[J]. Journal of Shanxi Agricultural University (Natural Science Edition), 2017, 37(1): 72-76. DOI:10.3969/j.issn.1671-8151.2017.01.013 |
| [34] |
Li J, Huang B, Wang Q X, et al. Effects of fumigation with metam-so dium on soil microbial biomass, respiration, nitrogen transformation, bacterial community diversity and genes encoding key enzymes in volved in nitrogen cycling[J]. Science of the Total Environment, 2017, 598: 1027-1036. DOI:10.1016/j.scitotenv.2017.02.058 |
| [35] |
Zheng W, Zhao Z Y, Gong Q L, et al. Responses of fungal–bacterial community and network to organic inputs vary among different spatial habitats in soil[J]. Soil Biology and Biochemistry, 2018, 125: 54-63. DOI:10.1016/j.soilbio.2018.06.029 |
| [36] |
Adams R I, Miletto M, Taylor J W, et al. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limita tion at short distances[J]. The ISME Journal, 2013, 7(7): 1262-1273. DOI:10.1038/ismej.2013.28 |
| [37] |
Nguyen N H, Song Z, Bates S T, et al. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild[J]. Fungal Ecology, 2016, 20: 241-248. DOI:10.1016/j.funeco.2015.06.006 |
| [38] |
Ferrigo D, Mondin M, Ladurner E, et al. Effect of seed biopriming with Trichoderma harzianum strain INAT11 on Fusarium ear rot and Gibberella ear rot diseases[J]. Biological Control, 2020, 147: 104286. DOI:10.1016/j.biocontrol.2020.104286 |
| [39] |
王晨骄, 陈颖, 李蕾, 等. 兼具苯酚降解和禾谷镰刀菌生防功能菌的活性验证[J]. 农业环境科学学报, 2020, 39(11): 2548-2553. WANG Chen-jiao, CHEN Ying, LI Lei, et al. Activity verification of a bacterium capable of facultative phenol degradation and biocontrol of Fusarium graminearum[J]. Journal of Agro-Environment Science, 2020, 39(11): 2548-2553. DOI:10.11654/jaes.2020-0409 |
| [40] |
张广旭, 吕亭辉, 周娣, 等. 黄瓜连作土壤高温处理对根结线虫和枯萎病的影响[J]. 植物营养与肥料学报, 2019, 25(6): 917-924. ZHANG Guang-xu, LÜ Ting-hui, ZHOU Di, et al. Effects of high-temperature treatment on root knot nematode and Fusarium wilt in continuous cucumber cropping soil[J]. Journal of Plant Nutrition and Fertilizers, 2019, 25(6): 917-924. |
| [41] |
Zhou X G, WU F Z. Differentially expressed transcripts from cucum ber(Cucumis sativus L.)rootupon inoculation with Fusarium oxysporum f. sp. cucumerinum owen[J]. Physiological and Molecular Plant Pathology, 2009, 74: 142-150. DOI:10.1016/j.pmpp.2009.10.005 |
| [42] |
李君. 两种熏蒸剂对土壤微生物多样性和群落结构的影响[D]. 北京: 中国农业科学院, 2017: 45-46. LI Jun. Effects of two fumi gants on soil microbial diversity and community structure[D]. Beijing: Chinese Academy of Agricultural Sciences, 2017: 45-46. |
| [43] |
Kumar R, Kundu A, Dutta A, et al. Chemo-profiling of bioactive me tabolites from Chaetomium globosum for biocontrol of Sclerotinia rot and plant growth promotion[J]. Fungal Biology, 2020, 125(3): 167-176. |
| [44] |
Shanthiyaa V, Saravanakumar D, Rajendran L, et al. Use of Chaetomium globosum for biocontrol of potato late blight disease[J]. Crop Protection, 2013, 52: 33-38. DOI:10.1016/j.cropro.2013.05.006 |
| [45] |
Zhang D Q, Yan D D, Fang W S, et al. Chloropicrin alternated with biofumigation increases crop yield and modifies soil bacterial and fun gal communities in strawberry production[J]. Science of the Total Environment, 2019, 675: 615-622. DOI:10.1016/j.scitotenv.2019.04.222 |
| [46] |
Raheja Y, Kaur B, Falco M, et al. Secretome analysis of Talaromyces emersonii reveals distinct CAZymes profile and enhanced cellulase production through response surface methodology[J]. Industrial Crops and Products, 2020, 152: 112554. DOI:10.1016/j.indcrop.2020.112554 |
| [47] |
檀兴燕. 强还原土壤灭菌法缓解番茄连作障碍的效果及其土壤微生物群落的响应机制[D]. 淮北: 淮北师范大学, 2019: 15-52. TAN Xing-yan. Effect of reductive soil disinfestation on alleviating continuous cropping obstacles of tomato and response mechanism of soil microbial community[D]. Huaibei: Huaibei Normal University, 2019: 15-52. |
| [48] |
El Aamri L, Hafidi M, Scordino F, et al. Arthrographis curvata and Rhodosporidium babjevae as new potential fungal lipase producers for biotechnological applications[J]. Brazilian Archives of Biology and Technology, 2020, 63: 2020. |
| [49] |
于一雷, 徐卫刚, 郭嘉, 等. 石油烃污染底泥的理化性质及酶活性研究[J]. 生态环境学报, 2020, 29(5): 1021-1030. YU Yi-lei, XU Wei-gang, GUO Jia, et al. Study on physicochemical properties and enzyme activity of petroleum hydrocarbon contaminated sediment[J]. Ecology and Environmental Sciences, 2020, 29(5): 1021-1030. |
| [50] |
Gupta D K, Chattoo B B. Leucothecium emdenii: A novel larvicidal fungus against mosquito larvae[J]. Journal of Invertebrate Pathology, 1999, 73(2): 226-227. DOI:10.1006/jipa.1998.4755 |
 2021, Vol. 40
2021, Vol. 40